Background: Bacterial infections pose a major challenge to risk management activities in the area of chronic haemodialysis, as vascular access-related infections are the main cause of mortality among these patients. Methods: Prospective surveillance study lasting 7 months (March-September, 2008) at two haemodialysis units in a district health area Gran Canaria, Spain. We used the methodology proposed by CDC´s Dialysis Surveillance Network. Results: 1545 patients/month were recorded, 60.5% with an arteriovenous fistula (AVF), 35.5% with a permanent catheter (PC), 3.0% with grafts and 1.0% with temporary catheters. The rate of adverse events was 8.6 cases per 100 patients/month, 9.1 for AVF patients, and 2.9 for PC. Nevertheless, the other types of infections (respiratory, urinary tract, skin and chronic ulcers) showed similar rates. Microbiological cultures were taken in 82.2%, but this rate increased to 91.0% when a vascular access-related infection was suspected. Empirical treatment was adjusted to antibiogram results in 90.0% of occasions. A low incidence of multi-resistant microbes was observed. Gram-positive and gram-negative bacteria appeared in similar proportions. Conclusions: Vascular access is the main risk factor for infectious events. Epidemiological surveillance has allowed us to detect areas of improvement in different settings, acting as a key element in risk management and patient safety.
Introducción: Las infecciones bacterianas representan un gran desafío en las estrategias de gestión del riesgo, prevención y seguridad de los pacientes en hemodiálisis; entre ellas, las infecciones del acceso vascular (AV) suponen la primera causa de morbimortalidad. Métodos: Estudio de siete meses de duración sobre la incidencia de determinados eventos adversos e infecciones (marzo-septiembre de 2008) en las unidades de hemodiálisis del Área Sanitaria Sur de Gran Canaria (hospital y centro periférico) mediante el empleo de la metodología del Dialysis Surveillance Network de los Centers for Diseases and Control (CDC) de los Estados Unidos. Resultados: Se incluyeron 1.545 pacientes/mes, un 60,5% con fístulas AV (FAV), un 35,5% conectados a catéteres permanentes (CP), un 3,0% tratados con prótesis y un 1,0% con catéteres temporales. La incidencia de eventos fue 8,6 casos por 100 pacientes-mes, y fue de 9,1 para las FAV y de 2,9 para los CP, mientras que las tasas de otras infecciones (respiratorias, de herida o de orina) fueron similares. Se pidieron cultivos antes del tratamiento antibiótico en el 82,2% de los casos, más si la sospecha era de bacteriemia y/o de infección AV (91,0%). El 9,0% de tratamientos se ajustaron con antibiograma. Destaca una baja incidencia de bacterias mutirresistentes, mientras que las infecciones relacionadas con el AV fueron causadas en una proporción similar por bacterias grampositivas y gramnegativas. Conclusiones: El AV es el principal factor de riesgo para el desarrollo de infecciones. La vigilancia epidemiológica he permitido detectar oportunidades de mejora en ámbitos asistenciales distintos, y se integra como elemento fundamental en el desarrollo de estrategias multidisciplinarias de seguridad del paciente.
INTRODUCTION
Health care-related adverse events are the subject of much attention in all health institutions, and morbidity and mortality for nosocomial infections have a significant impact on this issue.1 Chronic renal failure (CRF) patients who are on haemodialysis have a high risk of contracting infections due to the technical complexity of the health care they receive as well as the immunosuppressive state they usually find themselves in. These infections are the second-leading cause of death among haemodialysis patients, with an attributed mortality rate of 14%.2 Especially important are infections in vascular access points, which are the leading cause of bacteraemia and loss of the vascular access in these patients.3
The type of vascular access (VA) directly affects the risk of developing infections and is the most important risk factor in the development of bacteraemia and VA infection, which from lowest risk to highest are: arteriovenous fistulas (AVF), endovascular prostheses, tunnelled catheters, and non-tunnelled catheters.4-9 The frequency with which VA are used varies according to the characteristics of the health system and the population being treated. For example, haemodialysis patients with a high prevalence of diabetes tend to have a lower rate of AVF and a greater use of catheters.8 In the United States, where the first VA placed in a patient was a permanent catheter to be later replaced by an endovascular prosthesis (graft), the “Fistula First” campaign has been recently instated in order to raise awareness on and promote AVF as the vascular access of choice and to increase the probability that patients receive the safest type of VA.11,12 At the same time, widespread use of antibiotics has led to the potential problem of microbial resistance, and haemodialysis has historically been one of the health care fields in which the appearance of new resistant strains have been observed for the first time.13-15
Epidemiological surveillance of infectious events and antibiotic resistance can help understand the baseline state of a health care field and can provide important information when developing plans for improving and implementing future measures for control. It can also provide data for evaluating the possible impact of the activities meant to prevent and control bacterial resistance.4,16,17
In 1999, the Centres for Disease Control (CDC) in the United States implemented the first epidemiological surveillance system for haemodialysis, known as the Dialysis Surveillance Network (DSN),2,3 which was consolidated in the recently created National Healthcare Safety Network (NHSN). In Europe, this type of surveillance is less frequent, since it was only in 2006 that the creation of multicentre systems was organised, with the production of standardised guidelines, indicators, and recommendations.18-23
Within this context, we took interest in the implementation of a surveillance system for bacterial infections in haemodialysis patients in the southern region of Gran Canaria, with the objective of quantifying and analysing the epidemiological characteristics of (infectious and non-infectious) adverse events and to identify possible opportunities for improvement.
METHODOLOGY
Prospective study on the incidence of certain adverse events and infections, applying the methodology used by the CDC through the DSN, including all (chronic) haemodialysis patients, with stratification based on the type of vascular access.2 The study period was six months, from March to September of 2008. The patients under surveillance were treated in the haemodialysis unit of the tertiary Hospital Universitario Insular de Gran Canaria (Gran Canaria Island University Hospital) and at a peripheral health centre (Avericum) in the southern region of Gran Canaria. Patients were treated at one centre or the other based on clinical criteria. Patients underwent dialysis from Monday to Saturday on morning or afternoon shifts, with a total of 79 available workstations distributed in the following manner:
1. Hospital. 25 workstations: 16 in the general treatment room, four in a room for hepatitis C virus (HCV), two in a room for Hepatitis B virus (HBV), two in a room for human immunodeficiency virus (HIV), and one in isolation.
2. Avericum. 54 workstations: 50 in a general treatment room and four in a room for patients with HCV.
We obtained indicators in the following manner:
1. Denominator (census): we obtained our data from those patients that received haemodialysis during the first two days of each month, stratifying them by type of VA. In this way, we were able to safely estimate the number of patient/month, since the patients are always the same and rotate in fixed shifts. The total number of patients treated during the first two days of each month produced the number of patients for that month. Using this information for the study period, we produced a value for the total number of patients per month, which was stratified by type of VA. We used this formula because of the low internal variability of patients on haemodialysis and in order to facilitate data collection by the department staff.
2. Numerator (incident cases): a case was any patient that required hospitalisation and/or antibiotic treatment and/or produced a positive blood culture.
a) Hospitalisation was defined as when the dialysis patient was admitted to any hospital department for over 24 hours due to any reason; a case file was produced for each hospitalisation.
b) Each time that systemic antibiotic treatment was administered to these patients, a case file was created, except for instances during the hospitalisation process, since this was considered as the same event. Repeated treatments of the same antibiotic treatment within a 21-day period were considered as the same case.
c) In the case of positive blood cultures, even in the absence of hospitalisation or antibiotic treatment, a case file was produced. A positive blood culture test 21 days after the previous one was also considered a new case.
3. Interpretation of the indicators: the rates observed show the mean percentage of patients that register a new case every month. For example, a rate of 3.0% during the month of January indicates that an average of 3% of patients had an adverse event during that month.
For patients that developed repeated VA-related infections over time, the first, second, third, and even fourth events were considered, according to the data collection protocol established by the NHSN.3
Data collection and analysis
During the first week of each month over the course of the surveillance period, the nursing staff at both centres compiled all of the necessary census information, indicating the total number of patients that received dialysis, stratified by type of vascular access.
For each individual case, the nephrology staff created a new epidemiological case file. Each month, the case files were sent to the study coordinator in the preventive medicine department, where the data were reviewed and stored in the computer file.
A case file was produced for all infectious and non-infectious problems associated with the VA (thrombosis, haemorrhage, etc.), death, and cardiovascular problems, whereas hospitalisations for more than 24 hours that were due to any other cause were classified as incident cases in the “other” category.
In case of an infectious pathology, the attending physician recorded the diagnosis in the case file, and the final classification of the infection was performed by the surveillance coordinator in the preventive medicine department, according to the CDC16 criteria for the following infections: VA infections: local infections and with secondary bacteraemia (catheter-related), bacteraemia (non-catheter related), skin and soft tissue infections (non-surgical), pneumonia, and urinary tract infections (UTI).
In the data analysis section, we calculated the frequencies of categorical variables and used chi-square tests for comparing proportions using SPSS software, version 17.
RESULTS
We amassed a total of 1545 patient/month, with a monthly mean of 221 patients, although with major differences between the two dialysis centres, both in the number and type of VA, as expressed in Table 1. The distribution of types of VA was different between the two centres, with a greater proportion of AVF than permanent catheters in patients attended at the peripheral health centre, and an inverse relationship observed at the hospital, where permanent catheters were more common. They all were Hickman type catheters, reaching 71.5% of the total. A very low proportion of the VA were prostheses or non-tunnelled temporary catheters (both were less than 5% of the total).
Incident cases
We observed a total of 134 incident cases and the majority of them (53.7%) were for antibiotic treatment administration. The rate of incident cases (expressed as 100 patient/month) varied significantly between the two different health centres and, above all, between the different types of vascular access used. Rates were much higher in patients attended at the hospital and in those with higher-risk types of vascular access (Table 2).
Non-infectious events
The rates of adverse events varied greatly, but the risk gradient for type of VA was maintained, except for those cases classified as “other,” which were much frequent in patients with AVF. The rate of lost vascular access points varied widely based on the type of access used. Throughout the surveillance period, we observed the loss of two AVF and one endovascular prosthesis due to non-infectious causes.
Infectious events
We identified 41 infectious events related to the vascular access, with an incidence of 8.6 cases per 100 patient/month, 33 cases of bacteraemia related to the vascular access, and 8 cases of local infection. These infectious events caused the loss of 9 VA (seven permanent catheters and two temporary ones). As shown in Table 2, the rates of infectious events varied according to the type of vascular access used, especially in the infections of the VA, whereas the rate of infections not related to the VA (wounds, UTI, and respiratory infections) were similar among different types.
Use of antibiotics and microbiological cultures
In all, we administered 66 different systemic antibiotic treatments (in monotherapy or combined), and the global percentage of microbiological cultures taken before starting treatment was 73.2%. In the hospital, cultures were taken in 82.2% of treated cases, and in 91% if there was a suspicion of bacteraemia and/or an infected VA, whereas cultures were taken in only 56.7% of cases in which antibiotic treatment was administered in the peripheral centre, with 90% of VA-related infections being diagnosed. Cultures were not taken in cases of repeated infectious event in the same patient (second, third, and fourth events). When diagnosing a patient with a wound infection, cultures were taken in 70% of cases before prescribing treatment, in 50% of cases of UTI, and in none of the cases of respiratory infections. In 78% of cases, empirical treatment was given with vancomycin and ceftazidime when a VA-related infection was suspected, and was adjusted according to an antibiogram in 90% of cases. The overall rate of specific use of vancomycin was 4.4% patient/month in the hospital, and 0.8% in the peripheral centre; these rates also varied by type of VA (Table 2).
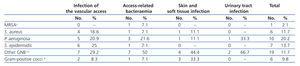
Table 3 shows the bacterial isolations by infection type (only for first events, not including repeated cultures of the same microorganism). We isolated multi-resistant microorganisms in only four cases, and the proportion of infections caused by gram-positive bacteria was similar to that of gram-negative bacteria, both in VA-related infections and others.
DISCUSSION
We performed a surveillance study of the appearance of adverse events in a chronic haemodialysis population. In Spain little experience has been gained in this field, and so this study could contribute to understanding the situation within this population.18,24 Compared with data from Spain and Europe in general,7,24 our health care area uses a 20% lower proportion of AVF (mean in Spain: 79.5%) and endovascular prostheses (mean: 10.5%), whereas permanent catheters are used 3.5 times more frequently (35.5% vs the rate in Spain of 9.9%).
The frequency of using VA varied between the two centres. Patients with an AVF or a permanent catheter accounted for 60.5% of all such patients, and the frequency of using AVF was greater in the peripheral centre (71.5%) than in the hospital (34%), whereas permanent catheters had a similar distribution in the two health centres. On the other hand, the use of prostheses and temporary catheters was quite low, and so the data regarding patients with these types of accesses may not have been representative so we have not shown their results. This is not an ideal situation, taking into account that the majority of scientific societies and medical authorities recommend the AVF, given its lower rate of occurrence of adverse events.5-12,19-22,26-28
The rates of adverse events were higher in patients with permanent catheters than in those with fistulas or prostheses, both in the peripheral and central hospital centres, coinciding with results from other authors around the world.3,7-9,25,29 The rate of hospitalisation is twice as high in the group of patients with permanent catheters than in the AVF group, as well as the rate of infections related to the vascular access point (both in local infections and bacteraemias).
In patients on haemodialysis in the United States, the rates of catheter-related bacteraemias is estimated at 0.9-2.0 events per patient-year.2 Similarly, surveillance data indicate that hospitalisation rates have increased by 29% due to bacteraemia, and 24% due to cellulitis, since 1993.3 Ferrero et al,29 in a similar study carried out in Italy, observed a 0.18% rate of bacteraemia associated with the vascular access, which is a lower rate than that presented by the NHSN3,25 and the values we observed (1.3 and 4.4 episodes per 100 patients/month in patients with AVF and permanent catheters, respectively).
Non-infectious adverse events had a similar pattern to infectious events, with greater rates for permanent catheters and patients treated at the hospital.
With regard to antibiotic treatment and laboratory use, the data indicate a good overall situation, with a low incidence of multi-resistant bacteria and a rational use of health resources (antibiotic agents and microbiological cultures). However, there is room for improvement, especially with regard to antibiotic treatment without previous culture, such as in the case of respiratory infections. The rate of use of vancomycin was between 0.1 and 1.9 per 100 patients/month in patients with AVF, and between 2.8 and 5.5 for patients with permanent catheters, which are acceptable results when compared with the 2006 data from the DSN,3 falling below the 75th percentile. In our health care field, this type of empirical treatment, adjusted to the antibiogram results, continues to be the treatment of choice, taking into consideration the low incidence of multi-resistant bacteria and the absence of bacteria resistant to vancomycin.
Our study has some limitations. In the first place, it had a descriptive design, and so we cannot estimate risk. We cannot conclude that the presence of an AVF causes a lower risk of infectious events, since this would require a more analytical study design (experimental, cohorts, or case/control). Even so, the approximations made using the epidemiological surveillance design used here can be useful, especially because our results are consistent with those from other studies.3,24,28 Also, the study period was quite short, only seven months, although we believe that the sample size of 1545 patient/month may offer a good estimation of the incidence of adverse events. However, we did not establish a predetermined sample size, since the primary objective of the study was to detect problems associated with the treatment of patients on chronic haemodialysis. Finally, we must point out that the scarce experience with this type of study in our field has revealed errors in the estimation of parameters.
Nevertheless, our study has detected some areas for improvement, such as the lack of cultures taken in respiratory infections. However, the high rate of use of permanent long-term catheters compared to AVF represents the most critical point in morbidity and mortality, health costs, quality of health care, and patient safety, which is not dependent on the workers in our haemodialysis units, since we also depend on health professionals from other fields, such as vascular surgeons and hospital administrators, as stated in the “Change package overview” promoted by the “Fistula First” campaign in the USA.11 Our data show that the increased risk of an adverse event is also higher in our area (without basing ourselves solely on data from previous studies), and should be used to motivate health care professionals that work in treating these patients to increase the rate of AVF as the VA of choice and to reduce the overall use of permanent catheters such as a long-term VA.
The implication of hospital administrators and vascular surgeons in this matter is considered indispensable in order to achieve these objectives.
The surveillance programme that we presented here is easy to implement, consumes few resources, and is well accepted by health care professionals working in haemodialysis units. Moreover, it provides useful information for introducing improvement and control measures, and that have been demonstrated to reduce the rates of infections and improve the use of antibiotics.3,24,29 Finally, our results can be useful for the planning and coordination of clinical management of haemodialysis patients, becoming a key factor in the development of multi-disciplinary strategies for ensuring patient safety.1,3,16-19
Table 1. Population characteristics and rate of events by type of vascular access
Table 2. Rates of adverse events expressed per 100 patients/month
Table 3. Microorganisms isolated by type of infection