Background: Ischaemia-reperfusion is one of the main causes of kidney complications. The most frequent lesion is acute tubular necrosis. Ozone oxidative preconditioning exerts a modulatory effect of redox state of renal cells in models of ischaemia-reperfusion, by stimulating endogenous antioxidant mechanisms. Similar results have been obtained in more recent studies using ischaemic postconditioning. Objectives: To evaluate the effect of ozone oxidative postconditioning on renal function and morphology in an ischaemia-reperfusion rat model. Methods: We used forty female Wistar rats weighing between 150g-200g randomly divided into 4 groups (negative control, positive control, oxygen and ozone). The groups: positive control, oxygen and ozone were subjected to 60 minutes of ischaemia and 10 days of reperfusion. During reperfusion, the oxygen group was given 26mg/kg body weight of oxygen, and the ozone group 0.5mg/kg body weight of ozone, rectally. At the end of the experiment urine and blood samples were taken for renal function tests and kidneys were removed for histological study. Results: The ozone group showed no significant differences for filtration fraction and proteinuria compared to the negative control group. The glomerular filtrate rate, renal plasma flow and creatinine showed a slight improvement in comparison with oxygen and positive control groups. The ozone group showed significantly less overall histological damage than the positive control and oxygen groups. Conclusions: Ozone postconditioning showed to have a protective effect in preserving renal function and morphology.
Antecedentes: La isquemia-reperfusión es causa fundamental de complicaciones renales. La lesión más frecuente es la necrosis tubular aguda (NTA). En modelos de isquemia-reperfusión se ha demostrado que el precondicionamiento oxidativo con ozono ejerce un efecto modulador del estado redox de las células renales, al estimular los mecanismos antioxidantes endógenos. Trabajos más recientes, que han empleado el postcondicionamiento isquémico, han obtenido resultados similares. Objetivos: Evaluar el efecto del poscondicionamiento oxidativo con ozono sobre la morfología y la función renal en un modelo de isquemia-reperfusión en ratas. Métodos: Se utilizaron 40 ratas Wistar hembras con un peso entre 150 y 200 g, divididas al azar en cuatro grupos (control negativo, control positivo, oxígeno y ozono). Los grupos control positivo, oxígeno y ozono fueron sometidos a 60 minutos de isquemia y 10 días de reperfusión. Durante la reperfusión al grupo oxígeno se le administraron 26 mg/kg de peso corporal de oxígeno y al grupo ozono, 0,5 mg/kg de peso corporal de ozono, por vía rectal. Al final del experimento se tomaron muestras de orina y de sangre para las pruebas de función renal y se extrajeron los riñones para el estudio histológico. Resultados: El grupo ozono no mostró diferencias significativas en los valores de fracción de filtración y proteinuria con respecto al grupo control negativo. Los valores de intensidad de filtrado glomerular, flujo plasmático renal y creatinina mostraron una mejoría ligera en comparación con los grupos oxígeno y control positivo. El grupo ozono mostró de forma significativa un menor daño histológico global que los grupos control positivo y oxígeno. Conclusiones: El poscondicionamiento con ozono tuvo un efecto protector en la preservación de la función y de la morfología renal.
INTRODUCTION
Renal ischaemia-reperfusion (I/R) can be observed in several disorders, which may compromise blood flow in the renal artery.1 I/R is one of the main causes of kidney complications.2 The most common injury is acute tubular necrosis (ATN), which increases when ischaemia time exceeds 30 minutes, reflecting that they are determining factors in the kidney’s viability once reoxygenation is reestablished.3-6
Ischaemia contributes to endothelial dysfunction,7 which creates an imbalance between vasodilator agents and vasoconstrictor agents, as more of the latter are produced. This results in an uncontrolled vasoconstriction,3-5 mainly in the afferent arterioles and glomerular capillaries, which reduces the filtration area and affects the glomerular filtration rate in the same way.3 The increase of vascular resistance in the afferent and efferent arterioles changes the renal plasma flow (RPF) and reduces the glomerular filtration rate (GFR) even more. During the reperfusion, a turbulent blood flow is created that may affect the endothelial walls due to the shear force on the vascular intima, making the previous changes even worse in the post-ischaemic kidney.1,8
Tubular dysfunction develops as a result of the reduction in adenosine triphosphate (ATP) and oxygen deprivation alters the cellular balance,8 producing apoptosis and autophagy, which irrevocably compromise the organ’s function and patient’s life.
Alterations in the tubular epithelium cause an inflammatory process which extends to the systemic level and leads to deterioration of the blood flow and organ damage. Furthermore, the tubules become obstructed with the epithelial cell debris, and as a result, the hydrostatic pressure in Bowman’s capsule increases, causing GFR to decrease.1,7,9
During the I/R, reactive oxygen species (ROS) production has an important role, which causes an imbalance between the prooxidant and antioxidant systems, the former’s activity being predominant. This is known as oxidative stress.10
Ozone oxidative preconditioning has a modulatory effect of the tubular cells’ redox state, by stimulating the endogenous antioxidant mechanisms.5,11 More recent studies have obtained similar results using ozone postconditioning12,13 and ischaemic postconditioning.14,15
This aim of this study is to evaluate the ozone oxidative postconditioning effect on renal function and morphology in an I/R rat model.
MATERIAL AND METHODS
We used 40 young female Wistar rats, weighing between 150g and 200g. The procedures performed were in accordance with those approved by international animal welfare committees, in agreement with the regulations established for animal experimentation (ICILAS, 2000).
We separated the rats into four groups: negative control (sham), positive control (subjected to I/R), oxygen (subjected to I/R with oxygen treatment) and ozone (subjected to I/R with ozone treatment).
All animals were anaesthetised with intraperitoneal sodium pentobarbital at a dose of 30mg/kg body weight. They were also administered heparin at 50UI/100g body weight through the same route. A laparotomy was performed by making a midline longitudinal incision until the peritoneal cavity. Once having located the kidney, we dissected and exposed the renal vascular bundles and placed haemostatic clamps for 60 minutes to induce an ischaemic kidney injury on all of the animals, except for the negative control group. We then sutured the incision and followed for 10 days.
Every day 26mg/kg body weight of oxygen was applied to the oxygen group, and 0.5mg/kg body weight of ozone was applied to the ozone group. This treatment was carried out through a rectal cannula, every day for 10 days. The ozone was produced using a medical ozone generator, Ozomed, in the Centro de Investigaciones del Ozono (Ozone Research Centre) Havana, Cuba. The ozone was obtained from therapeutic oxygen and was used immediately after it had been generated.5,12,13
The rats were treated 12 hours after the surgical interventions had been performed to avoid interactions between the anaesthetic treatment and the ozone or oxygen. Having applied the treatment, the animals were confined to metabolic cages for 24 hours. Urine samples were taken for the following measurements. We conducted compartmental analysis of the para-aminohippuric acid (PAH) and inulin plasma clearance to determine the renal plasma flow (RPF) and GFR.
Blood was extracted by means of an intracardiac puncture to calculate the plasma creatinine concentration and later a xiphopubic laparotomy was performed and an abdominal separator positioned. The kidneys were located and a bilateral nephrectomy was performed for histological analysis.
Renal function tests
Procedure to conduct compartmental analysis of plasma clearance
We administered heparin at 100UI/100g body weight to the rats. They were anaesthetised with intraperitoneal sodium pentobarbital at 30mg/kg body weight. We shaved both inguinal areas and made an oblique incision. Both vascular nerve bundles were dissected and we cannulated the left femoral vein. We administered 0.8ml of a 12mg/ml PAH solution and the same volume of a 2mg/ml inulin solution, so that these substances would reach adequate plasma levels. After, we dissected and cannulated the right femoral artery. Zero time point was set upon administration of the first bolus, and then 0.2ml of blood was extracted every 10 minutes until 10 samples were taken.13,16
Techniques to measure para-aminohippuric acid and inulin concentrations in plasma
Plasma was obtained and subject to a deproteinisation process using cadmium sulphate to measure the PAH and inulin.
To measure PAH, we followed the Bratton and Marshall17 photo-colorimeter technique, modified by Smith.18 Inulin was measured using the direct resorcinol method,17 with no alkaline treatment.
These clearances were calculated using the multi-compartment decomposition analysis of the disappearance curves corresponding to the plasma substances, using mathematical MATLAB software, version 6.5 for Windows.15,16
Microanalytical technique to measure the plasma creatinine concentration
Plasma was obtained and it was deproteinised using the sodium tungstate method, modified by Brot and Sirata,19 using the photo-colorimeter technique.20
Measuring protein concentrations
Protein concentrations were measured using the Biuret photo-colorimeter technique.21
Histopathological study
Histological processing
The kidneys were cut along the sagittal plane through the hilum. The segments were fixed in a 10% neutral buffered formalin solution (sodium phosphate buffer (0.01mM/l, pH 7.4) and then the conventional paraffin inclusion method was used. Cross-sections of 3µm thick were made and two conventional staining methods used: haematoxylin and eosin, and Periodic acid-Schiff (PAS). We conducted studies using a Nikon 50i light microscope, with an oil-immersion objective (1,000 X). Images were obtained using a high resolution DA-5M-U1 digital camera connected to the microscope.
Morphological and morphometric analysis
We examined a histological sample of the right kidney’s vertical hemisection to evaluate the overall histological damage.
We examined 10 fields in the cortical and medullary regions for each sample, which extended from each kidney’s upper pole to the lower one. An average of 30 to 35 proximal tubules was examined per animal, resulting in a total of 150 to 165 tubules per study group. We only studied the tubules that were transversally cut, determining whether the following were present: cell vacuolation; apoptotic nuclei, ballooned or pyknotic; cell necrosis; discontinuity of tubular basement membrane (TBM); denudation of TBM; cell desquamation; brush border; cylinders in the lumen; and peritubular inflammatory cells.
We used a programme developed for the .Net platform to quantify these variables, using the Visual Studio 2008 environment, C# language, in which 0 was considered the normal histological structure and 1 the pathological conditions of each variable.22
We assigned different values to each of the variables so as to assess the overall histological damage, in accordance with its contribution to tubular damage: (4: cell necrosis, cell desquamation and TBM denudation; 3: loss of brush border and cellular vacuolation; 2: cylinders in the lumen, peritubular inflammatory cells and TBM discontinuity; 1: apoptotic nuclei, ballooned or pyknotic). We considered 8 to be the minimum value for irreversible damage. Using the programme we calculated the percentage of damaged tubules per field and obtained 10 overall histological values for each animal.
We used Graph Pad Prism, version 5.0 for Windows for the statistical processing. In all cases, we calculated the average and standard deviation using descriptive statistics. We compared the percentage of damaged tubules per field between the groups. We performed the Kruskal-Wallis non-parametric tests and Dunn’s multiple comparison test to analyse the differences between the groups.
RESULTS
Renal function tests
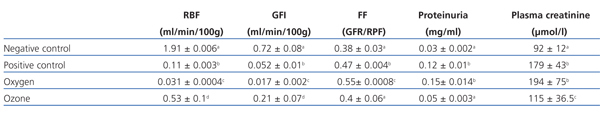
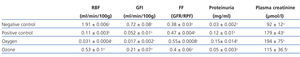
RPF and GFR significantly decreased in the positive control group in comparison to the negative control group. There were no statistically significant differences between the positive control and the oxygen groups. RPF did not reduce in the ozone group as it did in the positive control and oxygen groups (Table 1).
The positive control and oxygen groups’ filtration fraction (FF) increased and the highest values were found in the oxygen group. Ozone postconditioning did not change the FF value in comparison to the negative control group (Table 1).
There were no significant differences between the negative control and ozone groups with regards proteinuria, but there was between the other groups (Table 1).
We observed significant differences in creatinine behaviour in all groups compared to the negative control group, although the ozone group improved but did not reach normal values. There were no statistically significant differences between the positive control and the oxygen groups (Table 1).
Histopathological study
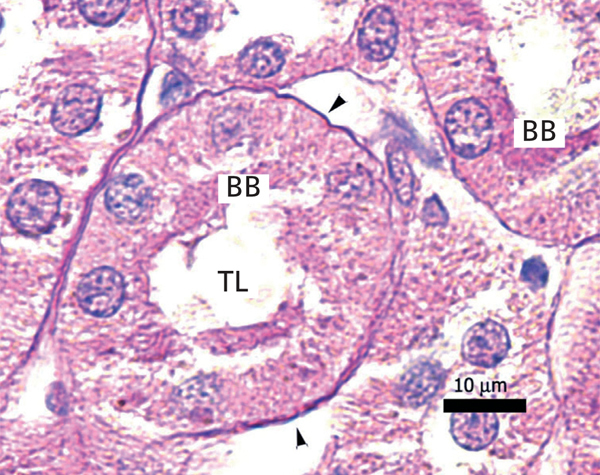
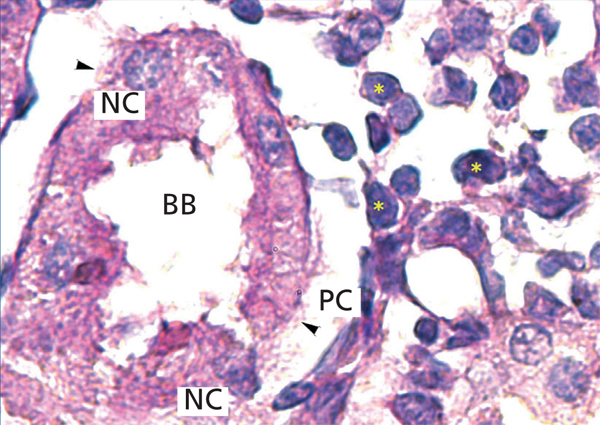
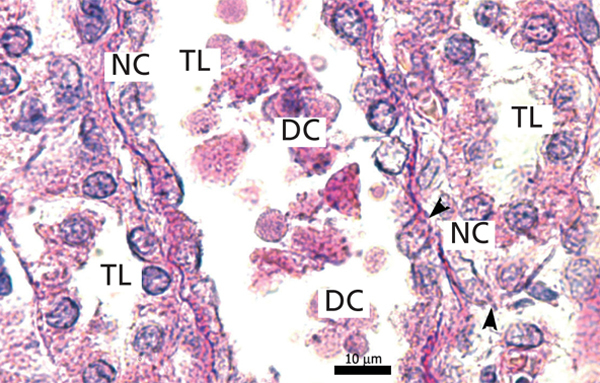
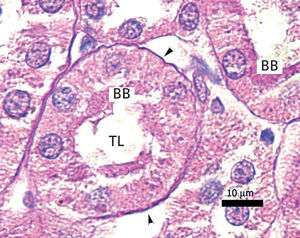
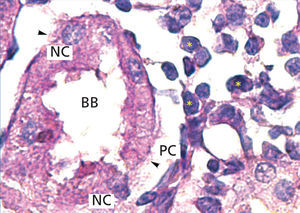
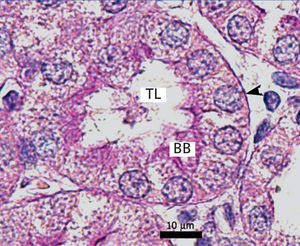
The proximal tubules’ structure in the negative control group was normal (Figure 1). Significant damage was observed to the kidneys from the positive control and oxygen groups (loss of brush border, tubular dilation, epithelial cell necrosis, formation of cylinders in the tubular lumen, interruption and/or effacement of the TBM) (Figures 2 and 3).
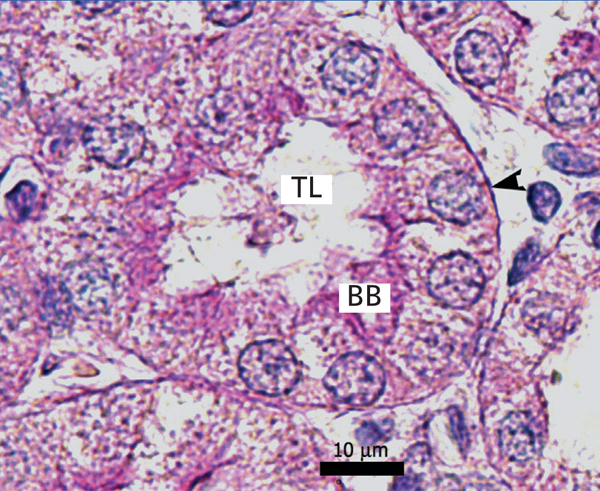
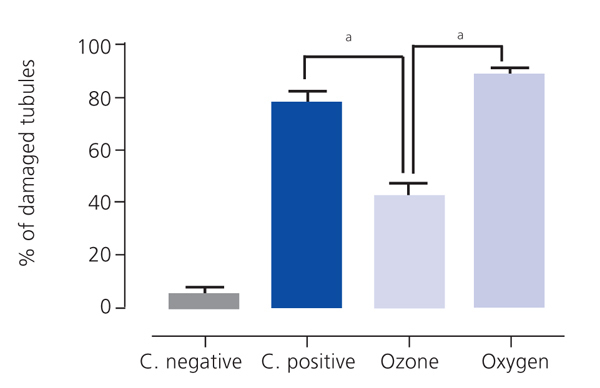
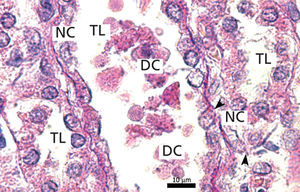
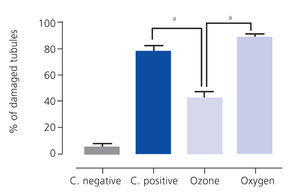
Ozone postconditioning caused recovery of tubular morphology. The proximal tubules’ brush border and other cell structures were better preserved (Figure 4). The animals from the ozone group showed significantly less overall histological damage than the positive control and oxygen groups (Figure 5).
DISCUSSION
Renal function tests
The significant reduction of RPF and GFR values in the positive control compared to the negative control group are likely to be due to ischaemia-induced endothelial damage,12,13 which is worsened by the shear forces, increasing endothelial dysfunction and ROS formation even more.3
Ischaemia causes renin release to increase, consequently increasing angiotensin formation; all of these cause RPF to remain low following reperfusion, therefore reducing GF,23 to which angiotensin also contributes, given its impact on reducing the filtration coefficient by acting on the mesangial cells.1,3 Furthermore, the ROS change the filtration barrier integrity,10,22 given that they attack many of its components such as type IV collagen, proteoglycans and sialoproteins.24-26
There were no statistically significant differences between the positive control and the oxygen groups, which showed that the oxygen did not influence renal function recovery, given that the oxygen intensified ROS formation.12
PAH and inulin clearance was produced in the ozone group with values that were much higher than in the positive and oxygen groups. This effect could have been caused by the ozone’s direct impact on tissue oxygenation, given that it improves erythrocyte rheology, increasing its flexibility and electrical charge,7 which in turn reduces blood’s viscosity and platelet aggregation, improving RPF in the kidney subjected to ischaemia.6,12,14
The RPF did not reduce in the ozone group as it did in the positive control and oxygen groups, i.e., less plasma fraction was filtered. This causes the plasma’s colloid osmotic pressure to increase in the glomerular capillaries less significantly than in the positive control and oxygen groups and, therefore, it has a reduced inhibitor effect on the GFR.7,23
Proteinuria is an indicator of kidney damage. Proteinuria in the ozone group was not significantly different from negative control animals showing the ozone’s antioxidant protection, decreasing the proinflammatory interleukins which are found in the inflammation produced by ROS.23 When this inflammation is produced in the glomerulus, the size of the molecules that are filtered can no longer be distinguished,25-27 and, as a result, high molecular weight globulins and albumin appear in the filtered product in quantities close to those in the plasma, as observed in the positive control and oxygen groups.4,23,28
Creatinine is a substance that depends on GF for its clearance.24 The ozone group recovered its glomerular filtration rate, in comparison to the other groups, which is shown by the creatinine excretion behaviour, and therefore its plasma concentration. Oxygen does not influence function recovery.
Histopathological study
Loss of the brush border, tubular dilation, epithelial cell necrosis, formation of cylinder in the tubular lumen and the interruption and/or effacement of the TBM found in the positive control group represent I/R-induced morphological kidney damage markers.27,29
Ozone postconditioning caused tubular morphology recovery, and as a result, this group preserved renal function better. It is considered that the renal tissue in the ozone group better supported I/R under our experimental conditions, given the ozone’s antioxidant characteristics which preserve the advanced glycation end product (AGE) tissue levels. This is in line with Calunga et al’s results.12
Our histological results coincide with those in the literature on the effect of ischaemic postconditioning in organs subjected to I/R.12,14
CONCLUSIONS
Ozone postconditioning showed a protective effect in preserving the renal function and reducing overall histological damage induced by I/R in the proximal tubules.
Ozone postconditioning has proven to be effective, meaning that it could be applied in cases of I/R-induced kidney damage. This type of treatment has an advantage regarding pre-determining factors, meaning that it could be applied in unforeseen clinical situations.
Funding
Instituto de Ciencias Básicas y Preclínicas Victoria de Girón (Victoria de Girón Institute of Basic and Preclinical Sciences), Departamento de Fisiología (Physiology Department), Laboratorio de Fisiología Renal (Kidney Physiology Laboratory).
Table 1. Effect of ozone oxidative postconditioning based on renal function evidence
Figure 1. Negative control animal¿s proximal tubule in the medullary and cortical regions. PAS staining
Figure 2. Positive control animal¿s proximal tubule in the medullary and cortical regions. PAS staining
Figure 3. Ozone-treated animal¿s proximal tubule in the medullary and cortical regions. PAS staining
Figure 4. Oxygen-treated animal¿s proximal tubule segments in the medullary and cortical regions. PAS staining
Figure 5. Overall histological damage