The effects of cinacalcet in persistent and/or hypercalcaemia-associated secondary hyperparathyroidism (SHPT) have been described in patients on dialysis.
ObjectivesTo evaluate the efficacy and safety of cinacalcet in SHPT not on dialysis and its effects on bone turnover markers.
MethodsNon-randomised, longitudinal, observational, analytical study of patients with chronic kidney disease (CKD) and SHPT (PTH >80pg/mL) as well as normo- or hypercalcaemia (≥8.5mg/dL), treated with cinacalcet.
ResultsMean cinacalcet dose was 30mg/day in 66.7%. We studied 15 patients (10 women), aged 66.0±17.93 years. The aetiology was unknown in 20% of cases. Sociodemographic variables and renal function parameters were recorded. We compared values at baseline as well as after 6 and 12 months. Calcium (10.3±0.55 vs. 9.4±1.04) and iPTH (392.4±317.65 vs. 141.8±59.26) levels decreased. Increased levels of phosphorus (3.7±1.06 vs. 3.9±0.85) and β-CTX (884.2±797.22 vs. 1053.6±999.00) were detected, although there were no significant changes in GFR, urinary calcium or other bone markers. Two patients withdrew from the study (gastrointestinal intolerance and parathyroidectomy, respectively).
ConclusionsCinacalcet at low doses is effective in the management of SHPT in CKD patients who are not on dialysis. Its use reduces iPTH and calcaemia, without causing serious side effects or significant changes in renal function.
Los efectos de cinacalcet en el hiperparatiroidismo secundario (HPTS), persistente o asociado a hipercalcemia han sido descritos en pacientes en diálisis.
ObjetivosAnalizar la eficacia y seguridad de cinacalcet en HPTS no sometido a diálisis y sus efectos sobre marcadores de recambio óseo.
MétodosEstudio analítico observacional, no aleatorizado, longitudinal, de pacientes con enfermedad renal crónica (ERC) e HPTS (PTH > 80pg/mL); con normohipercalcemia (≥8,5mg/dL), tratados con cinacalcet.
ResultadosLa dosis media de cinacalcet fue de 30mg/día en un 66,7%. Estudiamos 15 pacientes (10 mujeres), con edad de 66,0±17,93 años. Etiología desconocida en 20% de los casos. Registramos variables sociodemográficas y parámetros de función renal. Comparamos valores basales, tras 6 y 12 meses. Descendieron los niveles de iPTH (392,4±317,65 vs. 141,8±59,26) y calcio (10,3±0,55 vs. 9,4±1,04). Aumentaron los valores de fósforo (3,7±1,06 vs. 3,9±0,85) y ß-CTX (884,2±797,22 vs. 1.053,6±999,00), sin variaciones significativas del FG, calciuria y demás marcadores óseos. Registrados 2 abandonos (intolerancia digestiva y paratiroidectomía, respectivamente).
ConclusionesCinacalcet a dosis bajas es eficaz en el manejo del HPTS del paciente con ERC no tratado mediante diálisis, al disminuir la iPTH y la calcemia, sin ocasionar efectos adversos graves ni variación significativa de la función renal.
Cinacalcet (Mimpara®) is currently the only calcimimetic with an approved indication for the treatment of secondary hyperparathyroidism (SHPT) in patients with chronic kidney disease (CKD) on dialysis,1–3 as well as in primary hyperparathyroidism (PHPT) caused by parathyroid adenoma or carcinoma in patients who have not undergone a parathyroidectomy, or with persistence of the disease following parathyroidectomy.2 Also, it has been used successfully in residual SHPT from a kidney transplant, although it does not a formal indication4 in these patients.
The calcimimetics acts as a positive allosteric modulator of the calcium-sensor receptors expressed in multiple tissues such as the parathyroid glands, kidneys, bone (especially in osteoclasts) and blood vessels.5 Its activation increases signal transduction, presumably inducing intracellular conformational changes and reducing the threshold for calcium sensitivity. At the glandular level, this translates to lower production and secretion of parathyroid hormone (PTH), which is essential in the management of resistant SHPT and SHPT associated with hypercalcaemia in patients in dialysis, in whom it has also shown modification of bone turnover markers.6,7
Less known are the effects of calcimimetics on patients with abnormal renal function not on dialysis, in whom mineral and bone disorder (MBD) associated with CKD is present.5–7 The latter does not strictly include only abnormalities in calcium, phosphorus, PTH and vitamin D; it also comprises abnormalities in bone remodelling, volume and resistance; as well as vascular and soft-tissue calcifications.7,8 In patients with CKD, the determination of collagen degradation products and classic bone turnover markers have greater utility than bone densitometry to predict the risk of fractures. Hence the importance of studying these parameters.7
This work aims to determine the efficacy and safety of cinacalcet in the treatment of SHPT in patients with CKD not treated with renal replacement therapy, in whom, owing to their serum calcium levels, the use of vitamin D and its derivatives is not safe, and in patients with severe hyperparathyroidism with a contraindication for surgery. It also aims to describe its effects on bone remodelling markers.
Patients and methodsWe conducted an observational, longitudinal study in a cohort of patients followed in our outpatient clinics. Patients enrolled had a diagnosis of SHPT (iPTH level>80pg/ml), whether with hypercalcaemia (which limited the use of both calcium-chelating agents and vitamin D and its analogues) or with normocalcaemia (corrected calcium ≥8.5mg/dl) resistant to these treatments. Patients were on stages 3–5 CKD (estimated glomerular filtration rate [eGFR] between 60 and <15ml/min/1.73m2).
The sociodemographic variables of the sample were recorded.
In each patient the use of cinacalcet for compassionate use was requested and authorised.
The patients started treatment with cinacalcet at a dose of 30mg in a single dose taken in the morning, and the dose was adjusted according to the evolution of the measured parameters.
The eGFR was calculated using the MDRD-4 formula, and iPTH, calcium, phosphorus, osteocalcin (OC), total alkaline phosphatase (AP), beta-crosslaps (β-CTX) and P1NP levels were determined using our laboratory's standard techniques.9 These variables were recorded at baseline, 6 months and 12 months from the start date of treatment with cinacalcet. We recorded adverse reactions and suspensions of this treatment, as well as associated medication based on phosphorus-chelating agents (both calcium and non-calcium), vitamin D analogues and biphosphonates.
The discrete variables were summarised using frequencies and percentages, and continuous variables were summarised using means±standard deviations (SDs), medians and percentiles (P25 and P75). The analysis was performed with the greatest number of subjects with observed and recorded data for each variable, since lost values were not replaced; in all cases it was always n≥9. We used Friedman's 2-way analysis to study the change in the variables at baseline, 6 months and 12 months, and the Wilcoxon signed-rank test for the change after a year. The statistical analyses were performed using the IBM® SPSS® Statistics v. 19 software program. The level of significance was set at p<0.05.
ResultsA total of 15 patients were enrolled; 10 were women, and the mean age was 66.0±17.9 years. They were distributed as CKD 3 (8 patients), CKD4 (3 patients) and CKD 5 (4 patients). The cause of CKD was vascular (7 patients), glomerular (3 patients), interstitial (one patient), diabetic (one patient) or of unknown origin (4 patients).
Before starting treatment with cinacalcet, 50.0% of patients were taking paricalcitol, 13.3% were taking non-calcium phosphorus-chelating agents and 26.6% were taking biphosphonates; these agents were continued. The starting dose of cinacalcet in most patients was 30mg/day, and after a year the mean dose of cinacalcet was 36.2±14.1mg/24h.
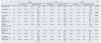
Table 1 shows the evolution of eGFR and bone and mineral metabolism parameters following the treatment with cinacalcet, as well as the results of the comparisons performed.
Evolution of estimated glomerular filtration rate and bone and mineral metabolism parameters in patients with secondary hyperparathyroidism due to CKD not treated with dialysis after cinacalce treatment: comparisons at baseline and at 6 and 12 months.
| N | Baseline | 6 months | 12 months | pa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (P25; P75) | Mean (SD) | Median (P25; P75) | Mean (SD) | Median (P25; P75) | |||||||||
| Creatinine (mg/dl) | 12 | 2.45 | (1.30) | 2.03 | (1.43; 3.57) | 2.51 | (1.52) | 1.68 | (1.21; 4.25) | 2.75 | (1.52) | 1.87 | (1.58; 4.32) | 0.099 |
| Urea (mg/dl) | 12 | 99.60 | (51.10) | 72 | (59; 133) | 93.67 | (51.10) | 72.0 | (59; 133) | 117.83 | (57.05) | 95 | (75.7; 138.2) | 0.117 |
| PTH (pg/ml) | 10 | 392.47 | (317.65) | 294 | (176.0; 556) | 293.1 | (464.6) | 146 | (110; 253) | 141.8 | (59.26) | 142.0 | (98.7; 178.8) | 0.009 |
| Serum calcium (mg/dl) | 14 | 10.3 | (0.55) | 10.45 | (10.1; 10.7) | 9.8 | (1.06) | 9.3 | (8.9; 10.6) | 9.37 | (1.04) | 9.3 | (8.5; 10.4) | 0.005 |
| Calciuria (mg/24h) | 10 | 0.16 | (0.18) | 0.09 | (0.7; 0.17) | 0.26 | (0.21) | 0.15 | (0.10; 0.48) | 0.18 | (0.90) | 0.21 | (0.1; 0.26) | 0.154 |
| Serum phosphorus (mg/dl) | 10 | 3.7 | (1.06) | 3.55 | (2.95; 4.32) | 3.71 | (0.67) | 3.65 | (2.97; 4.37) | 3.88 | (0.85) | 3.6 | (3.3; 4.4) | 0.011 |
| Estimated glomerular filtration rate (ml/min/1.73m2) | 11 | 29.78 | (15.35) | 28.81 | (14.4; 41.03) | 33.44 | (24.00) | 28.63 | (14.9; 48.1) | 31.02 | (17.42) | 33.28 | (14.3; 44.46) | 0.807 |
| Beta-crosslaps (pg/ml) | 11 | 884.92 | (797.2) | 616 | (248; 1309) | 1657.67 | (1291.71) | 1785 | (571; 2109) | 1053.63 | (999.0) | 763 | (461; 1129) | 0.041 |
| Osteocalcin (ng/ml) | 11 | 226.9 | (460.7) | 53.0 | (19; 219) | 122.1 | (91.32) | 140.0 | (23; 181) | 86.45 | (125.6) | 29 | (18; 83) | 0.919 |
| Total alkaline phosphatase (U/l) | 10 | 110.83 | (88.21) | 81.5 | (65.0; 126.8) | 133.85 | (86.67) | 121.0 | (91; 130.0) | 78.3 | (26.9) | 73.0 | (54.5; 100.7) | 0.833 |
| P1NP (ng/ml) | 10 | 149.08 | (166.0) | 99 | (42.5; 207.5) | 217.7 | (250.62) | 123.5 | (55.8; 282) | 126.9 | (132.6) | 82.5 | (46; 171) | 0.314 |
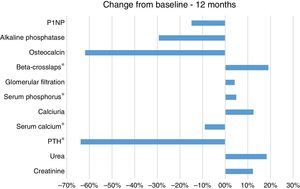
Before therapy the mean iPTH was 392.4±317.6, and mean blood calcium concentration was 10.3±0.5mg/dl. After 12 months of treatment he PTH levels decrease by 64% (Mean12 months=141.8) that was statistically significant as compared with baseline (Friedman, χ2(2)=8.222; p=0.016; Wilcoxon, Z=−2.599; p=0.009). The values of serum calcium decreased by 9.0% (Mean12 months=9.4mg/dl) this fall was significant as compared with baseline, (Friedman, χ2(2)=7.800; p=0.02; Wilcoxon, Z=−2.832; p=0.005) (Table 1 and Fig. 1).
We also observed a 5.0% increase in the mean serum phosphorus concentration after the one year of treatment (Mean12 months=3.9mg/dl) as compared with the baseline values (MeanBaseline=3.7mg/dl); this was not statistically significant according to Friedman, χ2(2)=5.250; p=0.072, although it was significant according to Wilcoxon, Z=−2.599; p=0.011. We also found a 19.0% increase in the levels of the bone resorption marker β-CTX after 12 months (Mean12months=1053pg/ml) compared with the baseline CTX values (MeanBaseline=885pg/ml); this change was significant according to the Wilcoxon rank test (Z=−2.045; p=0.041); but did not reach statistical significance according to Friedman, χ2(2)=1.000; p=0.607. There were no significant differences in the mean values for calciuria, eGFR, OC, P1NP or AP.
In two patients (13.3%) cinacalcet was discontinued: one due to gastrointestinal intolerance and the other patient because parathyroidectomy.
DiscussionOur pilot study has demonstrated that cinacalcet is effective in the management of SHPT associated with CKD in patients not yet in dialysis PTH levels decreased togheter with a reduction in serum calcium levels without detection of serious adverse events.
SHPT and MBD are present in patients with CKD.1,3,5–7 Both clinical conditions involve a substantial healthcare burden owing to their broad association with increase in cardiovascular risk, mortality and onset of fractures.3,5,8 Some studies even highlighted the differential involvement of each MBD factor in mortality (calcium/mortality>phosphorus/mortality>PTH/mortality ratio).3,8 In patients with CKD management of SHPT and MBD is made by controlling the triggering factors: through dietary restriction of phosphorus, the use of both calcium and non-calcium chelating agents, and correction of deficiencies such as vitamin D (or its analogues).3,6,10 On occasion, the above-mentioned measures are not effectve, and may be even have adverse effects as they increase the risk of vascular or soft-tissue calcifications in patients with high baseline blood calcium levels.5,11
Calcimimetics offer a therapeutic advantage as they act on the main physiopathological factors of both entities, which are already broadly related. Firstly, they reduce serum levels of PTH by decreasing its gene expression and secondly, they stimulate the synthesis of vitamin D receptors in the parathyroid gland, thereby increasing sensitivity to vitamini D with the subsequent PTH suppression.2,5,6,12,13 Some studies demonstrate tha cacimimetics stimulate calcitonin, which would cause a reduction in serum calcium levels. Regarding the serum phosphorus concentration, it is known that with GFR<60ml/min/1.73m2, there is an increase in the serum concentration of the phosphaturic hormone FGF-23, that also inhibits calcitriol synthesis.2,3 The increase in FGF23 is considered a physiological adaptation aimed to maintain better controlled phosphorus levels until advanced stages of CKD.3
There is limited informatio on the effect of cinacalcet in CKD outside of dialysis. To the best of our knowledge thera are two studies,4,5 neither of which investigates the effects of the drug in early stages of CKD or the effects on calcimimetics on bone remodelling. Therefore, our findings will be novel. We found that, after one year of cinacalcet in patients with stage 3–5 CKD (KDOQI), the serum iPTH levels significantly decreased, by around 65%, and serum calcium levels was reduced by almost 10% from baseline values. The above results match the observations in dialysis patients.1,5,12 There is considerable correlation between the reduction of PTH in dialysis and in CKD patients.1,3,11 However, there were discordant results with respect to serum levels of phosphorus and β-CTX which in dialyisi patients are normally reduced acter calcimimetics treatment.8,10–13 Therefore, the outcomes did not correspond to the respective 5% and 19% increases that our cohort presented. This latter results are probably explained by the reducton in PTH that deprive patients with significant GFR from the phosphaturic effects of PTH, in addition to the implication of cinacalcet in the reduction of FGF-23 levels.12
Bone remodelling markers are a dynamic reflection of the synthesis/degradation activity of the entire skeleton; this is different from densitometry/radiography, which focus on a static part of bone activity. Under normal conditions, the bone remodelling cycle last 3–6 months. Degradation of type I collagen results in blood and urine detection of both aminoxy terminal and carboxy terminal portions.14 There are more specific markers of bone formation, some are related to osteoblast activity (OC, P1NP and AP) and others are more related to bone resorption and osteoclast activity (ICTP, CTX and NTX).14 Their usefulness is based on the determination of the rate of bone turnover and risk of fractures and their function as a prognostic factor for response to treatment of metabolic bone diseases.7,14 However, to assess bone health, biopsy and tetracycline labelling remain the gold standard.7,14 β-CTX monitoring is useful in verifying response to antiresorptive therapy; the higher the initial values of this marker, the greater the response.1,6,7,12 The evolution of β-CTX levels did not correspond to what had been observed in previous works conducted in hemodialysis patients on cinacalcet1; however, we do not have studies in patients with CKD not in dialysis. It should be mentioned that around 27% of the patients included in our study were treated with antiresorptive agents, as they had GFR greater than 30ml/min. We are not certain whether there are implications associated with the use of cinacalcet and our findings. We believe that this subject should be further explored.
We did not observe any trends towards improvement of GFR, or changes in calciuria therefore the reduction in serum calcium concentration should not be related to urine losses.
Our conclusions may be limited by the small number of patients and the method of data collection which can lead to selection or information bias.
ConclusionCinacalcet effectively decreases iPTH and calcium levels in patients with CKD not in renal replacement therapy. Safety is similar to the observed in dialysis patients; no adverse effects occurred during the observation period. No significant variation in renal function was observed. It was observed an increase in serum phosphate and bone resorption marker β-CTX.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Orellana JM, Esteban RJ, Castilla YA, Fernández-Castillo R, Nozal-Fernández G, Esteban MA, et al. Uso de cinacalcet para el control del hiperparatiroidismo en pacientes con diferentes grados de insuficiencia renal. Nefrología. 2016;36:121–125.