For patients with chronic renal failure who develop secondary hyperparathyroidism (SHPT), imaging techniques can be useful, especially to evaluate the location, size and functional status of parathyroid glands. This review analyzes all available imaging procedures in the context of SHPT. We evaluate: 1) Cervical ultrasound (B-mode, Doppler, colour-Doppler and power-Doppler), 2) Scintigraphic studies (Tallium, 99mTc-MIBI and 99mTc-tetrofosmin), including non-standard image acquisition techniques (Pinhole, SPECT), 3) Positron emission tomography (PET), 4) Computed tomography (CT) and magnetic resonance imaging (MRI) and 5) hybrid scanners (SPECT/CT and PET/CT). Our recommendation is that SHPT patients who are initially non responders to medical therapy should be investigated using parathyroid scintigraphy and cervical ultrasound. 99mTc-MIBI uptake can be graded in a semiquantitative scale. Intense uptake indicates a low probability of success using medical treatment and parathyroidectomy should be considered. A moderate to faint uptake indicates that a more intensive medical therapy would probably be beneficial. In the case of no uptake of 99mTc-MIBI, PET should be performed. Where this is not available, MRI could be a possible alternative.
En los pacientes con enfermedad renal crónica que desarrollan hiperparatiroidismo secundario (HPTS), las técnicas de imagen pueden ser de utilidad, fundamentalmente para valorar la localización, el tamaño y el funcionalismo de las glándulas paratiroides. Esta revisión valora las técnicas de imagen de las que se dispone actualmente para evaluar las glándulas paratiroides en el contexto del HPTS. Se hace referencia a: 1) ecografía cervical (modo B, Doppler, Doppler-color y power-Doppler); 2) estudios gammagráficos (talio, 99mTc-MIBI y 99mTc-tetrofosmin), incluyendo técnicas especiales de adquisición de imágenes (Pinhole, SPECT); 3) estudios PET (tomografía por emisión de positrones); 4) tomografía computarizada (TC) y resonancia magnética, y 5) escáneres híbridos (SPECT/TC y PET/TC). Nuestra recomendación es practicar, en todos los pacientes con HPTS que no responden inicial y fácilmente al tratamiento médico, una gammagrafía con 99mTc-MIBI que puede complementarse con un Eco-Doppler color. Si la gammagrafía es positiva y, tras gradación de la intensidad de captación, alguna de las glándulas (no ectópicas) presenta un índice intenso, aunque se puede intentar intensificar el tratamiento, debería pensarse en la realización de una paratiroidectomía. Si la gammagrafía es positiva y, tras gradación de la intensidad de captación, ninguna de las glándulas (no ectópicas) presenta un índice intenso, debería intentarse la intensificación del tratamiento, y si no existe buena respuesta, considerar la paratiroidectomía. Si la gammagrafía es negativa, debería practicarse un PET si se dispone de dicha prueba. En caso de no disponer de PET, lo aconsejable sería realizar una resonancia magnética.
INTRODUCTION
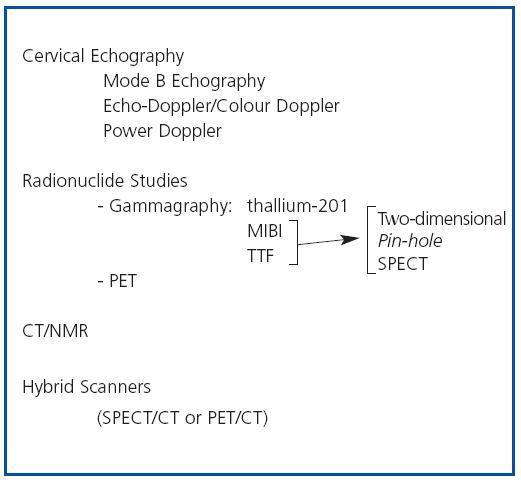
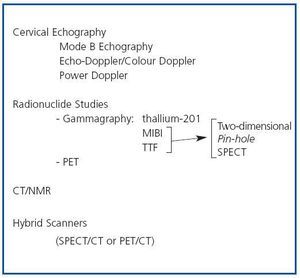
In patients with chronic kidney disease, who develop secondary hyperparathyroidism (SHPT), imaging techniques can be useful, basically to evaluate the location, size and function of the parathyroid glands. The embryology of the parathyroid glands is complex and a knowledge of their development and their possible anatomical locations can be useful when interpreting the findings of imaging techniques. Eighty five percent of the population have 4 parathyroid glands, two upper glands and two lower glands, which are usually situated behind the middle-upper third and the lower pole, respectively, of each of the two thyroid lobes.1 From 1 to 7% of individuals have only 3 parathyroid glands and from 3 to 13% have supernumerary parathyroid glands. In the latter case the glands are usually ectopic. This variation in number and location is due to differences in embryological development. The upper parathyroid glands originate in the fourth pharyngeal pouch of the thyroid gland and little displacement is required for them to adopt their definitive position, while the lower glands develop from the third pharyngeal pouch and migrate towards the pole. As a result of this greater embryological migration, the lower parathyroid glands may be more likely to adopt an ectopic position than the upper parathyroid glands. On average the parathyroid glands are 2-7mm long and 2- 4mm wide, and 0.5-2mm thick. The imaging techniques available for evaluating the parathyroid glands in patients with SHPT include the following (figure 1):
CERVICAL ECHOGRAPHY
Cervical echography was the first imaging technique used to study the parathyroid glands and it continues to be a test we can recommend, particularly in view of the fact that it is an economical and innocuous alternative, although it has the disadvantage of being very dependent on technical expertise and, consequently, its sensitivity can vary considerably. Colour Doppler linear multifrequency transducers (7.5- 12MHz) are used for studies of the parathyroid glands, although in patients who are obese or who have a multinodular goitre transducers with lower frequencies (5-7.5MHz) are employed.
Exploration Technique
When a scan is performed, the neck of the patient must be slightly stretched. Images are taken of each side of the neck in longitudinal and transversal sections, as well as from front to back and also with the head rotated sideways 45º. The thyroid gland is used as an anatomical reference, given that most pathological parathyroid glands will be found behind, at the sides of or below the thyroid gland. Other anatomical points of reference are the long muscle of the neck, the lower neurovascular bundle (lower thyroid artery and recurrent laryngeal nerve) and the oesophagus. The scan must cover the area from the submandibular gland to the subclavian vein and laterally from the tracheal midline to the carotid artery and jugular vein, as well as the area corresponding to the aortic arch, in order to exclude the presence of ectopic pathological glands
ECHOGRAPHIC DIAGNOSIS
Mode B Echography
While the first echographic scanners only permitted onedimensional images (mode A), with the development of brightness modulation (mode B), in which variations in the amplitude of pulsations are represented at each point by different shades of grey, it was possible to view an anatomical image. Normal parathyroid glands are not visible in mode B echography. This is due to their small size and the fact that their echogenicity is very similar to that of the thyroid gland. Enlarged parathyroid glands are perceived as a nodular, hypoechoic, homogenous image, which is rounded or oval in shape with smooth contours delimited by a fine echogenic line corresponding to the glandular capsule. When they are oval, their longitudinal axis is parallel to the longitudinal axis of the thyroid gland. In about 15- 20% of cases, the glandular echostructure may be heterogeneous, owing to the presence of necrosis and/or haemorrhage or even calcification, especially in very enlarged parathyroid glands. Various studies have demonstrated an excellent correlation between echographic measurements and the weight of resected glands and some of them have shown a correlation between glandular size and the severity and prognosis for secondary hyperparathyroidism and its response to treatment.3
Doppler and Colour Doppler Echography
Pulsed Doppler
The combination of echography and the Doppler technique enabled vascular structures to be studied, which allows velocities and flows to be estimated.
Colour Doppler
When colour-Doppler is used the data on velocities and frequencies can be converted to produce a colour image. Flow direction is expressed in red if it is positive and in blue if it is negative, irrespective of whether the vessel in question is an artery or a vein. With the colour Doppler technique pathological glands usually demonstrate a hypervascular pattern,4 the increase in vascularization showing an intraparenchymal distribution, with no perinodular component, although the latter is not always observed.
Power-Doppler Echography
This technique is based on the analysis of the amplitude of mobile echoes inside blood vessels, yielding contrasted vascular structures, and enabling smaller vessels to be identified, which not only facilitates their identification but also evaluates any vascularization. In this case the vascular pattern is the characteristic of an arterial bed with low resistance and a continuous flow is observed during diastolic movements. A recent study5 demonstrated that 60% of glands with no power Doppler result corresponded to diffuse hyperplasia or hyperplasia with initial nodularity, while 83.7% of glands which produced power Doppler readings presented nodular hyperplasia, even in parathyroid glands weighing 0.5g or less. An absence of vascularization has been described in approximately 10% of pathological parathyroid glands, owing to: 1) size less than 1cm; 2) deep location; 3) location close to large vessels, meaning there are transmission artefacts, and 4) the existence of areas of necrosis.
DIAGNOSTIC PROBLEMS
In general, the sensitivity and specificity of echography in parathyroid pathology studies are conditioned by various factors. The diagnostic sensitivity of echography is influenced by: a) the location of the pathological gland (atypical location, ectopic glands); b) their size (< 1cm); c) the coexistence of goitre and d) a history of cervical surgery. With respect to specificity, as well as the diagnostic problems which may occur with adenopathies of the cervical spine and thyroid nodules, there are two problems which are related to anatomical structures: the thyroid vein (it may cross the lower third of the thyroid lobe and be mistaken for an enlarged parathyroid gland) and the long muscle of the neck.
INDICATIONS
The classic indication for mode B echography in SHPT studies is the pre-surgical location of pathological glands (figure 2). Although in SHPT more than one gland is affected and, consequently, minimally invasive surgery is not applicable, the localization of the glands enables surgical exploration to be curtailed, thus reducing intervention time and surgical trauma. Colour Doppler imaging proves useful when, in mode B studies, there are diagnostic problems with thyroid nodules or cervical adenopathies. With power Doppler imaging arterial flow can be assessed and, consequently, to some extent, the degree of activity and then, from this, probably the response to treatment, although for this purpose gammagraphy would seem more advisable.
Echography-guided Interventions
The applications of echography as a guide to intervention procedures in SHPT include: 1. Fine-needle puncture aspiration (FNPA) 2. Percutaneous ablative treatment of adenomas or parathyroid gland hyperplasia FNPAs are of interest for characterizing nodular cervical lesions, when there is reason to suspect intrathyroid parathyroid glands. Percutaneous ablation with alcohol or even calcitriol6-8 consists of percutaneous injection of ethanol or calcitriol into the hyperplastic glands/adenomas, with the aim of reducing the PTH-producing mass. It may be indicated in patients with medical problems where surgery is contraindicated. In some centres it is currently used as an adjuvant to medical therapy. The size of the gland amenable to percutaneous ablation varies from one centre to another (generally > 5-10mm). The results described in recently published studies are much better than in early studies. Normal long-term serum PTH levels (1-3 years) have been described in approximately 80% of the patients who have been treated and the smaller the number of enlarged glands the better the results. Greater effectiveness has been reported for the alcoholization of less vascularized parathyroid glands than for glands which demonstrate a significant initial Doppler reading. The complications which may be associated with the procedure are primarily paralysis of the recurrent nerve, which is usually temporary, and peri-glandular fibrosis, which may make subsequent surgery difficult.
RADIONUCLIDE STUDIES
Gammagraphy
The first studies of parathyroid tissue functionality were conducted at the beginning of the 1980s using potassium analogues, such as thallium-201.9-11 Although gammagraphy using thallium-201 is still in use, nowadays other radiodrugs such as technetium-99m metoxyisobutyl- isonitrile (MIBI) or technetium-99m tetrofosmin (TTF) are used, chiefly because of the better physical properties of technetium-99m. In fact, MIBI is in the process of becoming the main radiodrug employed in the majority of nuclear medicine departments.
Radionuclides
Gammagraphy using Thallium (thallium-201)
Thallium is an analogue of potassium. It reaches the tissues in a manner which is proportionate to blood flow and it enters the cell actively, being transported across the cell membrane by the Na-K-ATPase pump. When gammagraphy with thallium is performed, the simultaneous thyroid uptake measured by technetium-99m thyroid gammagraphy needs to be subtracted from the results to avoid interferences from thyroid tissue.10 Although some initial studies have reported that thallium- 201 demonstrates good sensitivity in the identification of hyperplastic parathyroid glands,9,11 the quality of the images is substantially poorer than images obtained using TTF and MIBI.10
Double-phase MIBI Gammagraphy
Parathyroid double-phase MBI gammagraphy is the most widely used nuclear medicine technique for visualizing abnormal parathyroid glands. The compound used is hexakis isobutyl isonitrile (sestamibi) (MIBI). MIBI uses 99mTc as a marker, a radionuclide with a very short half-life, enabling high doses to be administered without exposing patients to high levels of radioactivity, which at the same time ensures better imaging quality and lower absorption by other tissues.12 The procedure entails the intravenous injection of 20mCi (740MBq) of MIBI. The images are obtained with the patient lying down, if possible with their neck stretched. Frontal images are obtained of an area which includes the neck and upper thorax. MIBI is taken up by both hyperfunctioning parathyroid and thyroid tissue, but, unlike thallium, there is a difference in its elimination from the hyperfunctioning thyroid and parathyroid glands, the process being more rapid in the thyroid glands. In a gammagraphy study an initial phase (thyroid phase) is obtained at 15 minutes and a late phase (parathyroid phase) at 60 minutes. Thyroid tissue has a physiological affinity during the initial phase, but clearance is rapid and normally any thyroid gland activity disappears. Normal parathyroid tissue shows no uptake in either of the two phases. Abnormal glands have a tendency to retain the tracer, which is visualized in the late phase. Thus, in cases of hyperplasia/adenoma, images are obtained of areas of hyperactive uptake activity, which are marked to a greater or lesser extent and are sometimes visible even in the initial phase image. Athyroid gland gammagraphy which is complementary to the MIBI gammagraphy is recommended in regions where goitre is endemic, in order to avoid false positives. The possibility of clearances in similar times for both tissues, which could lead to false negatives, has also been described.13,14 In MIBI gammagraphy, qualitative, quantitative or semiquantitative methods can be used to calculate the uptake index of pathological parathyroid glands: 1. Qualitative methods merely confirm the presence or absence of hyperfunctioning glands. 2. Quantitative methods include digital visualization, which corresponds to the late or parathyroid phase. The image is used to create a ROI (region of interest) in the parathyroid gland, which exhibits high uptake, and in the thyroid gland. The activity/pixel average in the parathyroid lesion is divided by the activity/pixel average of the thyroid gland to determine the increase in the activity of the parathyroid gland. 3. Semi-quantitative methods employ different grading options. Our experience using a semi-quantitative system has shown it to be very useful. The system awards a score of “0” to a lack of uptake, a score of “1” (mild) if uptake is similar to that of bone or soft tissue, a score of “2” (moderate) if the level of uptake is between 1 and that of the salivary glands, and a score of “3” (intense) if it is similar or higher than the salivary glands.15 This semi-quantitative system has been useful in helping us to decide which gland should be left in place or employed for an auto-transplant (the one with the least uptake) when a partial parathyroidectomy or a parathyroidectomy with an auto-transplant, respectively, is performed. It has also been very useful for evaluating the efficacy of the medical treatment of SHPT, both with vitamin D analogues and calcimimetic agents. And, finally, it has served as a means of predicting the results of medical treatment and for deciding whether to perform a parathyroidectomy (it is unlikely that a patient with an intense level of uptake will respond to medical treatment).15-17
Gammagraphy with Tetrofosmin
Tetrofosmin (TTF) is a molecule which is similar to MIBI and it has been proposed as a tracer for the pre-surgical detection and screening of abnormal parathyroid glands, although it is cleared more slowly in thyroid tissue than MIBI and the interpretation of double-phase images is more complex.18,19
SPECIAL TECHNIQUES FOR OBTAINING IMAGES GAMMAGRAPHY USING A PINHOLE COLLIMATOR
Gammagraphic images are usually visible in one plane (twodimensional), but there are special techniques which enable their definition and sensitivity to be improved. One of them involves using a Pinhole collimator, which takes the form of a cone and has the unique ability to produce a magnified inverted image. The addition of Pinhole images during the late phase of 99mTc-MIBI gammagraphy can increase the rate of detection of adenomas in indeterminate or initially negative studies.
Gammagraphy combined with the SPECT (Singlephoton Emission Computed Tomography) Technique
Gammagraphy with SPECT, which enables 360º images in three spatial planes to be obtained (three-dimensional images), has increased the ability of the method to locate the parathyroid glands and has permitted the detection of small lesions, which has given the technique greater precision. Some groups use SPECT together with the pin-hole technique, which results in a significant increase in sensitivity.20,21 However, the use of this technique in clinical practice is controversial, given that the pin-hole method is not suitable for the detection of ectopic glands located in the mediastinum.
Factors which Influence Radiotracer Uptake
Although the main factor affecting the localization of hyperfunctional parathyroid glands by nuclear medicine techniques seems to be related to their size, the comparison of morphological and functional data suggests that either TTF or MIBI gammagraphy not only reveals an increase in the size of glands but also identifies the presence of hyperfunction in parathyroid tissue. Small adenomas have been easy to identify but the downside is that false negatives have been described in fairly enlarged glands. Consequently, size is not the only factor which decides ease of detection. The mechanism by means of which the radionuclide remains for a long period of time in hyperfunctional glands is unknown, but it is probably the result of one or several circumstances, such as the phase of the cell cycle, the type of cell, mitochondrial density, the presence/absence of expression proteins, such as P-glycoprotein, and specific biochemical markers like PTHi and calcaemia.22 In general, the uptake of both MIBI and TTF is considered to be greater in cases of nodular hyperplasia than in diffuse hyperplasia. In patients with SHPT the intensity of the focal uptake of MIBI in the parathyroid glands was considered to be directly related to the phase of the cell cycle. Higher uptake levels correlate with the active phase of cell growth, indicating that MIBI gammagraphy reflects the functional state of the gland.23 The radiotracer seems to show more intense accumulation in glands that have large areas of oxyphilic cells which are rich in mitochondria than in areas with normal cell types.24 Some authors have found a correlation between serum calcium levels, PTH levels and numbers of oxyphilic cells in tissues, as well as between calcium levels and initial tracer uptake.24 Thus, serum calcium levels could play an important role in modifying the kinetic properties of MIBI by influencing membrane potentials. The level of radiotracer uptake has shown a significant correlation with the presence of greater numbers of mitochondria in the glands.25 The link which exists between MIBI uptake and retention and the number of mitochondria could also explain why uptake is greater in abnormal parathyroid tissue, this constituting an indicator of metabolic activity within cells. The relationship between the uptake of radiotracers and the expression of P-glycoprotein has been studied in hyperfunctional parathyroid glands. It has been noted that radiotracers are rapidly eliminated from parathyroid glands which contain P-glycoprotein and, therefore, the uptake seen in images will be negative. In parathyroid glands which do not contain P-glycoprotein the radiotracer stays in the cells, which makes it easier to detect them by gammagraphy.26-28 An increase in the expression of P-glycoprotein could be responsible for some false negatives in parathyroid gammagraphy. Similar results have been obtained using TTF. There is a significant correlation between MIBI uptake and serum PTHi values, but not with serum calcium, phosphorus, 25-OH vitamin D or 1.25-OH vitamin D levels.29 Calcium channel blockers, which are normally prescribed to treat hypertension, have been shown to reduce the secretion of PTH in vitro and to have an effect on the membrane potential of parathyroid cells, which could reduce their sensitivity to MIBI.30
Surgery Radioguided by MIBI Gammagraphy
By means of a gamma radiation detection probe which permits acoustic signals to be obtained and by measuring radioactive activity, a surgeon can be guided during an operation involving the excision of pathological thyroid glands. The technique is recommended for single adenomas which are visible in MIBI images without the presence of active thyroid nodules that could interfere with their detection, when there is no previous history of cervical irradiation or a family history of MEN.31 This technique is also indicated in patients who have been operated on more than once due to the persistence or recurrence of their hyperparathyroidism and when there is reason to suspect ectopic glands. Its most obvious advantages are the reduction in surgery time and the possibility of verifying the correct excision of parathyroid tissue, given that the radioactive activity of the tissue which has been surgically removed can be measured ex vivo.32
Positron Emission Tomography (PET)
PET is a non-invasive technique which enables images that reflect the metabolic activity of the parathyroid glands to be obtained. This technique employs isotopes which emit positrons (positive electrons). These isotopes generally have a very short average-life, which also means a reduction in the period of exposure and exploration. The agents used for PET offer better definition than those that are used for gammagraphy. Carbon-11 labelled with methionine is recommended for studying the parathyroid glands. Some studies have demonstrated a good correlation between 11C-methionine uptake and PTH and calcium levels.33-35 PET using 11C-methionine can be a useful method in patients with secondary hyperparathyroidism when the results of echography and gammagraphy have proved negative or inconclusive. Furthermore, ectopic glands are identified with greater precision by PET than by conventional gammagraphy, as it enables three-dimensional images to be obtained.
INDICATIONS
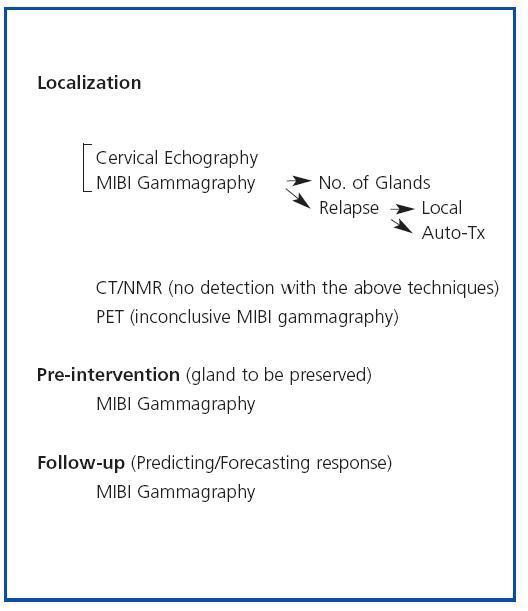
We can regard MIBI gammagraphy as our first option for identifying hyperfunctioning parathyroid glands (figure 2). Although SHPT patients who have not undergone surgical intervention may not need prior localization, owing to the high success rate of cervical exploration, the fact that the percentage of SHPT relapses (10-30%), the main cause of which continues to be the incomplete localization of the glands, is not insignificant needs to be considered. This is especially evident in patients who have repeat operations, in whom MIBI plays an important role in the detection of ectopic glands, relapses in situ or the hyperfunction of an auto-transplant.36 It may also be useful to decide the amount of gland which should be left or to employ for an auto-transplant when a partial parathyroidectomy or a total parathyroidectomy and an auto-transplant, respectively, are performed. In these cases it would be advisable to remove the glands with the highest uptake and leave less active glands, which would reduce the risk of relapse. It may also be useful to evaluate and/or predict the response to medical treatment. In the most recent studies the authors coincide in regarding echography and 99mTc-MIBI gammagraphy as complementary techniques and in recommending their combined use.37 PET should be reserved for locating parathyroid hyperplasia/adenoma when both echography and MIBI gammagraphy have produced negative results.
Computed Tomography and Nuclear Magnetic Resonance
Computed tomography (CT) can locate parathyroid glands which are less than 4mm in size, as well as peri-thyroid, substernal or retroclavicular soft tissue masses. Some series have reported that its sensitivity is similar to that of gammagraphy and echography in the diagnosis of multiglandular parathyroid disease, but the cost is greater and the detection of parathyroid tissue which is adjacent to the thyroid gland may be difficult.38 With the administration of intravenous contrast agent and the 0.5mm slices obtained by modern equipment, the sensitivity of the technique has improved, achieving a success rate of up to 80%. CT can detect ectopic parathyroid glands, although glands which are situated at the level of the shoulder or sternum are difficult to visualize because of the artefacts caused by bones, as it occurs in patients who have been operated on more than once, owing to the presence of metallic artefacts from previous operations. Nuclear magnetic resonance (NMR) seems to be more sensitive and, moreover, it offers the possibility of improving images by the use of contrast agent and 3-D reconstruction. Parathyroid adenomas have weak signals in T1-weighted images and strong signals in T2 images, which can be improved if gadolinium can be employed. Several studies refer to NMR as an imaging test which is more sensitive than CT,39,40 although it is more difficult to differentiate parathyroid adenomas from thyroid lesions.
Indications
Localization of parathyroid hyperplasia/adenoma when the above tests (echography and MIBI gammagraphy) have proved negative and the PET technique is not available.
HYBRID SCANNERS
The recent introduction of hybrid scanners for diagnostic purposes may have advantages compared to gammagraphy or PET alone. Both SPECT/CT and PET/CT provide images that merge the anatomical and functional modalities of the gland, which considerably improves the interpretation of the findings obtained for each of the techniques when they are used separately.41
Indications
Currently they are only indicated for the location of hyperfunctional glands when all the above techniques have failed.
KEY CONCEPTS
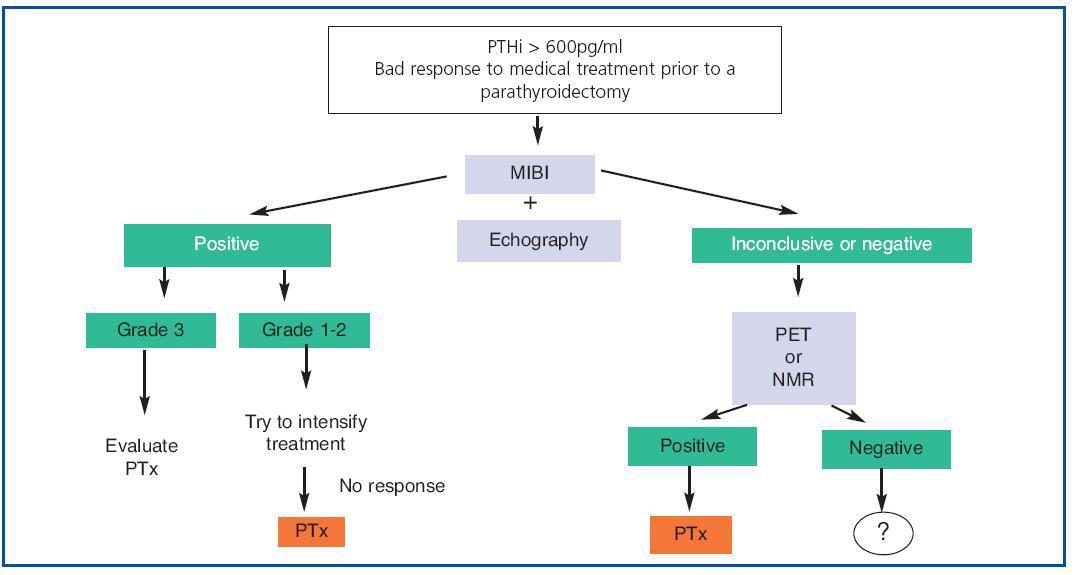
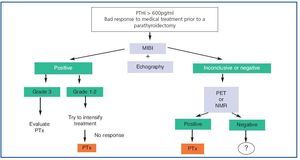
1. To sum up (figure 3), nowadays, in all patients with SHPT who do not respond readily to medical treatment at the outset, we recommend an MIBI gammagraphy which can be complemented with a colour Echo-Doppler scan.
2. If gammagraphy proves positive and, after measuring the intensity of uptake, any of the (non-ectopic) glands has an intense index (3), although an attempt can be made to intensify treatment, we should consider the possibility of a parathyroidectomy.
3. If the findings of the gammagraphy are positive, and, after measuring the intensity of uptake, any of the (non-ectopic) glands has an intense index (3), in other words they are grade 1 or 2, an attempt should be made to intensify treatment and, if the response is not good, a parathyroidectomy should be considered.
4. If the gammagraphy is negative, we should perform a PET or hybrid scan, if this is an option. If them are not available, an NMR scan is recommended. Once the gland(s) have been located, we would then propose a parathyroidectomy.
Figure 1. Imaging techniques in Secondary Hyperparathyroidism
Figure 2. Secondary Hyperparathyroidism Imaging Tables: Indications
Figure 3. Imaging techniques in Secondary Hyperparathyroidism: application algorithm.