Background: Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in patients with chronic kidney disease (CKD). Cardiovascular risk assessment in this population is hampered by the failure of traditional risk factors to fully account for the elevated CVD risk, mainly due to the reverse epidemiology effect, and the presence of risk factors specifically related to uremia. Hereby, we present the protocol of a prospective study aimed to assess the predictive value of imaging techniques and biomarkers for CVD in patients with CKD. Methods: From November 2009, 2.661 asymptomatic adult patients with stages 3-5D CKD will be recruited from nephrology services and dialysis units throughout Spain. Eight-hundred forty-three participants without CKD (control group) will be also recruited. During the follow-up, CVD events and mortality will be recorded from all CKD patients. One trained itinerant team will carry out a carotid ultrasound to assess intima-media thickness and presence of plaques. A composite atherosclerosis score will be constructed based on carotid ultrasound data and ankle-brachial index. Presence and type of calcifications will be assessed in carotid, femoral and brachial arteries, and in cardiac valves, by ultrasound. Finally, blood samples will be collected from all participants to study biomarkers. Discussion: The NEFRONA study will allow us to examine the usefulness of imaging techniques and biomarkers to assess atherosclerosis development and their predictive value in a Spanish population with CKD.
Justificación: La enfermedad cardiovascular (ECV) es la primera causa de mortalidad en pacientes con enfermedad renal crónica (ERC). La valoración del riesgo cardiovascular a partir de los factores tradicionales es poco útil en esta población debido al fenómeno de «reverse epidemiology» y a la existencia de factores específicos derivados de la uremia. En este trabajo presentamos el protocolo del proyecto NEFRONA, un estudio prospectivo con el objetivo de evaluar la utilidad de técnicas de imagen y biomarcadores en la predicción de la ECV en la ERC. Métodos: A partir de noviembre 2009 se reclutarán 2.661 adultos asintomáticos con ERC (estadios 3-5D) procedentes de consultas ambulatorias de nefrología y centros de diálisis distribuidos a lo largo del territorio español. Asimismo, se incluirán 843 participantes sin ERC (grupo control). Además, semestralmente se registrará la aparición de acontecimientos cardiovasculares y mortalidad. Un equipo itinerante realizará una ecografía carotídea para valorar el grosor íntima-media y la presencia de placas, y determinará el índice tobillo-brazo para la clasificación de la enfermedad ateromatosa. Para el estudio de las calcificaciones vasculares se utilizará un score basado en la presencia de calcificaciones en las arterias carótidas, femorales y braquiales, y en las válvulas cardíacas, mediante ecografía. Finalmente, se recogerán muestras de sangre para la determinación de biomarcadores. Discusión: El proyecto NEFRONA nos permitirá evaluar la utilidad de las técnicas de imagen y biomarcadores en la valoración de la enfermedad ateromatosa y su valor predictivo en la población española con ERC.
INTRODUCTION
Cardiovascular disease (CVD) is the main cause of death in chronic kidney disease (CKD).1 As a consequence, patients with CKD are considered as high-risk patients and are compared with patients with coronary heart disease.2 Different diagnostic algorithms are used in normal clinical practice to predict global cardiovascular risk, based on information provided by the common risk factors. However, in CKD, the prevention models extrapolated from the general population are not applicable due to the phenomenon of “reverse epidemiology”3 and the impact of specific factors associated with uraemia.4 As a result, it is necessary to use additional diagnostic instruments that help us predict cardiovascular risk beyond the traditional factors.5
From these techniques, carotid ultrasonography and the ankle-arm index (AAI) stand out because they are non-invasive reproducible low-cost tests that assess carotid atherosclerosis and the presence of a peripheral disease, respectively.6,7 Various prospective studies in the general population8,9 have shown conclusively that the increase in intima-media thickness (IMT) and/or in pathological AAI are risk factors irrespective of the CVD incidence rate. Until now, few prospective studies have examined the positive predictive value of IMT10-12 or AAI13,14 in patients with CKD. In this respect, the INVADE study10 recently analysed the positive predictive value of IMT in 3,364 patients with CKD. Despite the nature of this study, the reduced age range of the patients included in the study (starting at 55 years old), the lack of evaluation of patients receiving renal replacement treatment and the relatively short follow-up period (two years) were the main limitations of this study.10 Meanwhile, other authors have analysed the positive predictive value of IMT11,12 or AAI13,14 in patients receiving renal replacement treatment with dialysis. However, the results of those studies11-14 have not been conclusive, mainly due to the small sample size under consideration (< 100 patients) and the short follow-up period (12-15 months). As a result, to date, there are no comprehensive prospective studies that have evaluated the positive predictive value of the abovementioned techniques in patients with CKD, including all the stages of kidney disease. Furthermore, the study of the presence and localisation of calcium is possible through ultrasonography. In CKD, the presence of calcium in the arterial wall and the cardiac valves has been associated with an increase in morbidity and mortality due to CVD.15,16 However, the study of its positive predictive value, depending on the extent and the type of calcification (intimal as opposed to medial), continues to be unknown.17,18 As a result, the need to carry out an epidemiological study on the arterial pathology of patients with CKD is valid for determining the increased cardiovascular morbidity and mortality rate, as well as the lack of extensive epidemiological studies that have identified specific risk factors. In this study, we will assess the usefulness of imaging techniques together with the determination of biomarkers in the prediction of CVD in patients with CKD.
METHODS
Study design
The study is observational, prospective (a four-year follow-up) and multicentre. From November 2009 to April 2011, 2,661 patients with stages 3 to 5 CKD (estimated glomerular filtration rate < 60ml/min/1.73m2) will be recruited from outpatient nephrology clinics and dialysis centres across Spain. The selection of kidney patients will be carried out using a consecutive sampling of the patients arriving from outpatient nephrology clinics, which represent the entire health care system of the Spanish public network. Patients from both genders will be included, with ages ranging from 18 to 74 years, and those without a previous CVD. The patients who suffer from an intercurrent disease, which entails a lack of follow-up or a life expectancy of less than a year, will be excluded, as will those with a kidney transplant. Furthermore, 843 subjects without CKD (control group) will be included who will come from different Spanish community health centres, and for whom information will be gathered only from the baseline visit. The study protocol has been approved by the Ethics Committee of the Hospital of Arnau de Vilanova in Lleida, Spain.
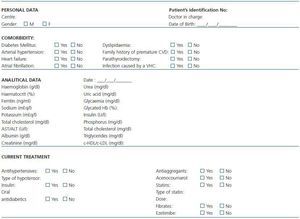
After obtaining informed consent, the medical history of all the participants will be studied, including socio-demographic variables, specific data of the kidney disease, the comorbidity and the current medical treatments (Table 1 and table 2). An itinerant team comprising two technicians and a nurse will record the tobacco use and alcohol consumption in grams/day and will carry out a physical examination that will include height in centimetres, weight in kilograms, waistline in centimetres, body mass index in kg/m2 and measurement of systolic and diastolic blood pressure in mercury millimetres (the mean of two determinations at a five-minute interval and with the person seated, using an automatic OMRON monitor).
Laboratory analysis
The systematic analytical determinations (complete blood count, biochemical test and lipid profile) (see the “Data analysis” section in Table 1) will be carried out either three months before or after the vascular examination. Furthermore, on the day of the vascular examination, blood samples will be taken from all the participants after six hours of fasting (in the instances in which this procedure is feasible) to obtain blood serum, plasma, DNA and RNA samples. These samples will be sent to the biobank of the Renal Research Network (REDinRen), where they will be processed according to the standard protocol and stored for the subsequent determination of biomarkers. An analysis will be carried out for cardiovascular biomarkers (homocysteine, lipoprotein[a], cardiotrophin, adiponectin, troponin T and cystatin C), inflammatory biomarkers (ultra-sensitive C-reactive protein, interleukin-6, interleukin-1, tumour necrosis factor-alpha, metalloproteinase [MMP] 2, 8, 9, 10 and metalloproteinase inhibitor [TIMP1]) and biomarkers related to mineral metabolism (FGF23, osteoprotegerin, osteocalcin, 25 [OH] vitamin D, 1.25 [OH]2 vitamin D and the ligand for receptor activator of nuclear factor Kappa-B [RANKL]). Furthermore, the following genetic polymorphisms will be determined: APOB_A618V, APOC3_3238C>G, APOE_C130R, APOE_R176C, CDKN2A/B_116191G>C, CXCL12_111738T>C, IL6_-174C>G, LPL_D9N, LPL_N291S, LPL_S447X and NOS3_i19342A>G.
Follow-up
The itinerant team will carry out follow-ups in the second and fourth years of the study. During these follow-ups the same procedures will be carried out as those in the baseline visit.
Cardiovascular morbidity and mortality
All the patients with CKD will be monitored every six months for a total of four years. In each examination the appearance of CVD will be recorded according to the ninth version of the International Statistical Classification of Diseases (ICD-9) which includes: angina pectoris, myocardial infarction, transient ischaemic attack, stroke, heart failure, arrhythmia, peripheral artery disease and aortic aneurysm. Furthermore, the patient’s cardiovascular cause of death will be registered (myocardial infarction, arrhythmia, heart failure, stroke, aortic aneurysm, mesenteric infarction and sudden cardiac death), as well as a non-cardiovascular cause (infections, tumours, accidents and kidney disease). All the data on the cases and mortality will be gathered by the nephrologist in charge of each centre.
Carotid ultrasonography
The ultrasound examination will be carried out using a BT09 Vivid i ultrasound device (General Electric) with an 8L-RS/13-6MHz linear transducer. The area of the carotid artery will be evaluated with the previously described protocol.19 The itinerant team will examine three predetermined segments of the arteries from both sides: common carotid artery (1cm proximal to the carotid bifurcation), bifurcation (1 to 2cm) and internal carotid artery (1cm distal to the bifurcation). The blood vessels will be studied using realtime ultrasonography and colour Doppler sonography through longitudinal and transversal sections, with a 45º rotation of the head towards the patient’s contralateral side and with the neck in a neutral anteroposterior position. The IMT and the atheroma plaques will be evaluated in each study. The IMT, defined as the existing distance between the lumen-intima interface of the carotid artery and the media-adventitia interface of the distal wall, will be determined in the three areas described. The plaques will be defined as the IMT focal-thickening with a height of > 1.2mm or more than 50% of the adjacent IMT. To identify them in the entire accessible carotid area, type B longitudinal and transversal sections will be carried out, followed by an examination with colour Doppler sonography, adjusting the technical parameters (gain, repetition frequency of pulses and range of speeds) to the existing speeds in the blood vessel. The reading process will be centralised, and two independent observers who are unfamiliar with the participants’ clinical characteristics will carry out the readings. For this reason, the GE EchoPAC equipment will be used with the IMT QIMT® automatic measurement software.
Ankle-Arm Index (AAI)
We will use a MD2 Huntleigh vascular Doppler probe with an 8MHz transducer and a cuff to manually measure blood pressure. The determination of the blood pressure will be carried out in the brachial artery of both arms and feet, ordinarily in the posterior tibial artery and the dorsalis pedis artery. To calculate the ankle-arm index (AAI), we will use either the highest brachial blood pressure measurement or the one closest to the time at which the malleolar pressure is taken.
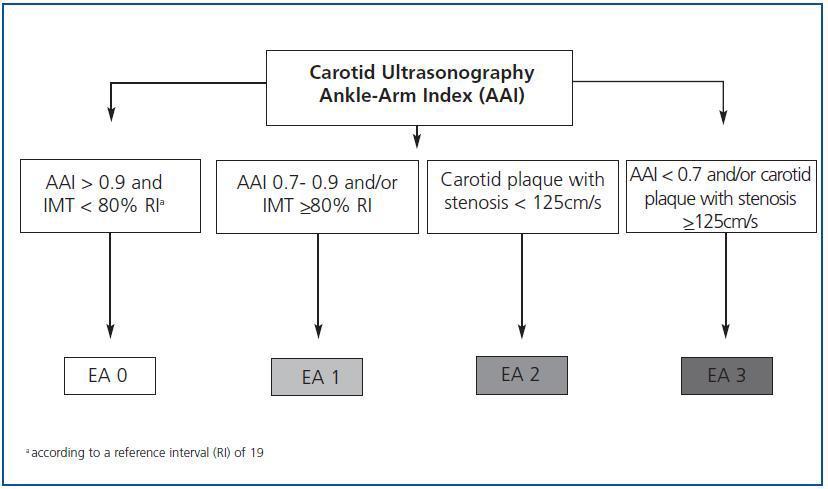
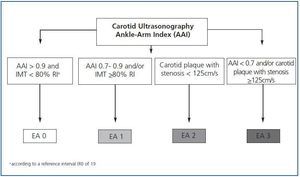
Classification of atherosclerosis
Except for the values obtained from the carotid ultrasonography and the AAI, we will classify the differences of atherosclerosis in four stages (figure 1):
Stage 0: subjects with AAI > 0.9 and carotid IMT < 80% according to the reference interval (RI).19
Stage 1: AAI between 0.7 and 0.9 and/or carotid IMT >80% according to the RI.
Stage 2: carotid plaque with stenosis < 125cm/s.
Stage 3: AAI < 0.7 and/or carotid plaque with stenosis >125cm/s.
Femoral ultrasonography
Ultrasonography of the femoral arteries will be carried out in order to identify the presence of atheroma plaques and calcifications in the vascular wall. It is expected that through this transversal study the common femoral artery (1cm proximal to the bifurcation), the femoral superficial artery (1cm distal to the bifurcation) and the presence of atheroma plaques will be identified anatomically.
Brachial ultrasonography
Brachial ultrasonography will be carried out where possible, in the extremity without vascular access. A longitudinal incision will be made in order to identify the presence of atheroma plaques, as well as the presence and localisation of calcium in the vascular wall.
Calcium Scoring
We will define calcium scoring as the presence/absence in each of the areas studied (carotid, femoral and brachial) and in all the areas examined (common carotid artery, bifurcation and internal carotid artery on both sides; common femoral artery and femoral superficial artery in both extremities and brachial artery). In accordance with the results of the ultrasonographies, we will use a scale from 0 (absence of calcifications in all the areas studied) to 11 (presence of calcium in all the areas studied). Furthermore, we will differentiate the localisation of calcium in each area studied: calcification of the intima, calcification of the media or both. The hyperechogenic plaques that produce echo shadows will be considered as vascular calcification (calcified plaques).
Echocardiogram
We will use a BT09 Vivid i ultrasound device (General Electric) with a 3S-RS vector transducer. The presence of left ventricular hypertrophy (LVH), the type of LVH (concentric, eccentric or concentric remodelling) and the presence of valvular calcifications will be assessed. The reading process will be centralised, and two independent observers who are unfamiliar with the participants’ clinical characteristics will carry out the readings.
Size of sample
The size of the sample of the patients with CKD has been calculated from data on the incidence rate of CVD described in this group and according to its stage.1 The calculation of the sample is based on the number of cases that need to be studied to obtain the minimum amount of informed consents to justify the results of the multivariate model for 15 variables. Certain errors I and II of 5% and 10%, respectively, have been assumed, as well as a 25% accumulated percentage of follow-up losses. It is expected that a total of 2,661 patients will be included, distributed in the following stages: 1,325 (stage 3), 713 (stages 4 and 5) and 623 in dialysis. Furthermore, 843 subjects without kidney disease (control group) will be included. The main objective of the control group is to detect important differences in the presence of plaques between kidney patients in each stage of the kidney disease and the subjects without kidney disease (data pending publication).
Statistical analysis
The data will be introduced in a database designed for this purpose and will be analysed using the SPSS v.17.0 statistical software. The absolute frequencies and the percentages with the qualitative variables will be presented, as well as the mean and the standard deviation for the quantitative variables that follow a normal distribution and average distribution and percentiles of 5 and 95 for the continuous variables that do not follow a normal distribution. The χ2 and the ANOVA tests will be carried out in order to study the comparisons between qualitative variables and/or quantitative variables, respectively. In the event where it is impossible to assume the normality of the distribution, we will apply the non-parametric Mann-Whitney U test or the Kruskal-Wallis test. The relationship between quantitative variables will be analysed using the calculation of Pearson’s rank correlation coefficient, or that of Spearman, according to their distribution. Concerning the association between the IMT or AAI values with the classic risk factors, multiple linear regression models will be used. The variables regarding risk factors will undergo adequate transformation so as to comply with the suppositions required by the linear regression technique, particularly when a non-linear relationship exists between the variables. With a view to the selection of the final model, the possible multicollinearity will be avoided by using a step-by-step algorithm for selection. The presence of plaques will be analysed using a logistic regression model. Furthermore, an analysis will be carried out on whether there are any differences between the control group and the CKD group in the variables that predict atheroma plaques by means of a separate estimation of the odds ratio. The longitudinal study will analyse the incidence rate of CVD, the cardiovascular mortality rate and mortality caused by other reasons, according to the parameters of the imaging techniques and the biomarkers studied, using the Cox regression model. The statistical significance will be considered as an alpha risk of 5% (p < 0.05).
DISCUSSION
The NEFRONA project is the first prospective study that will evaluate the usefulness of imaging techniques and biomarkers in the assessment of atherosclerosis, and its positive predictive value for attacks and mortality caused by CVD, in a large sample of kidney patients (approximately 3,000) in different stages, and which is a cross-section of patients in Spain. Given the paradoxical association between the traditional risk factors and the risk of CVD in the population with CKD, it is necessary to use other diagnostic instruments, such as imaging techniques and/or biomarkers, which allow an early identification of kidney patients with a high risk of presenting with a cardiovascular attack before it takes place.
We consider that establishing the prevention of CVD, based on an early diagnosis through the observation of the arterial wall (carotid ultrasonography), the presence and localisation of calcium in the arterial wall (carotid-femoral-brachial ultrasonography) and the valve structures (echocardiogram), as well as to determine the AAI and biomarkers, will allow us to:
1. Understand the natural history of arterial pathology and the specific risk factors in each stage of CKD.
2. Evaluate the impact of all the imaging parameters on the cardiovascular morbidity and mortality rates of these patients, and to set out standardised measurement and quantification criteria.
3. Authorise a standardised evaluation method of atherosclerosis that will facilitate its widespread use and the extrapolation of the results to other studies.
4. Identify the localisation of calcium in the arterial wall through ultrasonography, which will allow for the in-depth study of the pathophysiology of arterial calcification and its inducing factors.
5. Identify specific biomarkers that can complete the information obtained from the imaging techniques.
6. Finally analysing all these parameters will allow us to construct a mathematical model for the prediction of cardiovascular risk, which is different for each stage of CKD.
As a result, this study is expected to change the paradigm of prevention of CVD in patients with CKD through the practice of arterial ultrasonography and echocardiogram, the determination of AAI and of emerging biomarkers. These values will be very useful both for the comparison between the different stages of CKD and the subsequent comparison with the values obtained in a cohort group of patients without CKD (control group). However, to achieve the objectives set out, this project requires high statistical power and methodological rigour. We have therefore included most Spanish nephrology services in this study, and we have standardised the operation and evaluation procedures of the imaging techniques and the biomarkers through the implementation of all the imaging tests by an itinerant team, the creation of reading stations and the processing of the samples in a centralised biobank. As a result, we will construct a biobank of biological, genetic and imaging samples that are a cross-section of patients in Spain, which will be regulated by different research groups and which will allow us to carry out new analyses, including biomarkers that could be identified in the future.
Acknowledgements
This study was financed by Abbott Laboratories.
Table 1. Clinical data in patients with chronic kidney disease (CKD)
Table 2. Clinical data in patients without chronic kidney disease (control group)
Figure 1.