¿ Type of design and follow-up
Controlled and randomised, double-blind, multicentre trial with follow-up of 29 months.
¿ Assignment
Computer-generated permuted-block randomisation, in a 1:1 ratio, and stratified by the study centre, baseline proteinuria (proteinuria/creatinine > 1 in isolated urine sample) and history of cardiovascular disease (CVD).
¿ Masking
Double blind from outset.
¿ Scope
Multicentre study with patients from 623 centres in 24 countries.
¿ Patients
Inclusion criteria: 4,047 were included, all with type-2 diabetes and:
- Chronic kidney disease (CKD) with glomerular filtration (GF) estimated by the MDRD-4 equation of 20 to 60ml/min/1.73m2.
- Anaemia defined as haemoglobin (Hb) < 11g/dl.
- Transferrin saturation >15%
Exclusion criteria: uncontrolled arterial hypertension, previous renal transplant or with a programmed transplant from live donor current treatment with IV antiobiotics, chemotherapy or radiotherapy, neoplasia (except basal cell or squamous cell carcinoma), HIV infection, active bleeding, haemotological disease and pregnancy. Also excluded were patients who had had a cardiovascular event, gran mal seizure, major surgery or treatment with erythropoiesis stimulating agents (ESAs) in the 12 weeks prior to randomisation.
¿ Interventions
Random allocation to two groups, without prior lavage period:
- Subcutaneous darbepoetin alpha: 2,016 patients. Initially administered every 2 weeks at a dose of 0.75µg/kg and subsequently adjusted to obtain and maintain Hb at 13g/dl. The maximum monthly dose was preset at 600µg. After obtaining Hb of 12-13.5g/dl during two consecutive determinations, a monthly dose of darbepoetin was given.
- Placebo: 2,031 patients. For any figure of Hb < 9g/dl, a single subcutaneous dose of darbepoetin alpha was administered, 0.45µg/kg and subsequently the dose was adjusted every 2 weeks until Hb was >9g/dl, at which point the patient was again given a placebo.
Adjustments to the dose were made using an interactive voice response system which, in the case of the placebo group, simulated the changes that were made in the darbepoetin alpha group. After the adjustment of the initial dose, visits and Hb determinations were conducted monthly, and other analytic controls every 4 to 6 months. The events analysis was done at weeks 1, 13, 25 and subsequently every 24 weeks.
¿ Outcome variables
Primary:
- Time to first event, defined as the composite variable of death by any cause or cardiovascular event (MI, heart failure, ACVA or hospitalisation for myocardial ischemia), or:
- Time to composite variable of death by any cause or end-stage kidney disease (need for renal replacement treatment).
Secondary:
- Time until death.
- Cardiovascular mortality.
- Cardiovascular events.
- GFR decrease.
- Changes in two quality of life questionnaires from week 25: FACT-Fatigue (Functional Assessment of Cancer Therapy-Fatigue) and 36-Item Short-Form General Health Survey questionnaire (SF-36).
¿ Sample size
It was calculated that 1203 events would be necessary to detect, with a statistical power of 80%, a 20% risk reduction in the group treated with darbepoetin alpha, assuming an annual event rate of 12.5% in the placebo group, a 15% annual follow-up loss and attenuation in treatment effect due to the early use of erythropoiesis stimulating agents in patients who progressed to terminal renal failure. 0.048 was considered a type I error for the estimation of the sample size and intermediate analyses were made once 20, 40, 60 and 80% of the total expected cardiovascular events were registered.
¿ Statistical analysis
Intention to treat Kaplan-Meier curves were used and comparisons based on the log-rank test, stratified in terms of baseline proteinuria and the presence or absence of a history of cardiovascular disease. Hazard ratios and CI 95% were estimated with the stratified Cox proportional hazards model.
¿ Ethics and registration
Protocol approved by the ethics committees of each participating centre. Written informed consent was obtained from all patients. The trial was registered at ClinicalTrials.gov with number NCT00093015.
¿ Promotion
The trial was financed by Amgen. The authors made a conflict of interest statement. Two of them are employees of Amgen and the rest claim to have received fees for different activities from various pharmaceutical laboratories.
¿ PRINCIPAL RESULTS
Nine patients (four in the group treated with darbepoetin alpha and five in the placebo group) were eliminated from the analysis because their centres did not meet the Good Clinical Practice Guidelines.
BASELINE ANALYSIS OF GROUPS
The two groups had similar baseline characteristics. The mean age was 68 years, with 57.3% women. The median known duration time of diabetes was 15.4 years. 65.4% had previous cardiovascular disease.
The darbepoetin group had a lower proportion of heart failure than the control group (31.5 against 35.2%; p = 0.01). There were no differences in the remaining demographic, clinical or laboratory variables.
The median baseline Hb was 10.4g/dl (interquartile interval [IQ]: 9.8-10.9), transferrin saturation was 23% in both groups and ferritin 131 against 137µg/l. 41.8% against 42.7% received oral iron supplements, 1.4% against 1.6% intravenous iron and 8.8% against 10.2% had previously received an erythropoietin stimulating factor (darbepoetin group with respect to control group).
At the close of the database, there had been a follow-up loss of 153 patients (7.6%) from the darbepoetin group and 164 (8.1%) of the placebo group.
PRIMARY AND SECONDARY VARIABLES (table 1)
History of cardiovascular disease (RR 1.32; CI: 1.20-1-45) and proteinuria > 1mg/mg (protein quotient/creatinine in isolated urine sample) (RR 2.03; CI: 1.20-1.45) or of a first cardiovascular event, identified a population at greater risk of death, independently of the assigned group, treatment or placebo. Absence of treatment effect with darbepoetin was observed in each prespecified subgroup, with no significant interactions with age, sex, ethnic group, region or baseline GF.
In the follow-up of the groups:
1. The median Hb from the third month to end of study was 12.5g/dl (IQ: 12.0-12.8) in the darbepoetin group and 10.6g/dl (IQ: 9.9-11.3) in the placebo group.
2. The median monthly dose of darbepoetin in both groups was 176 µg (CI: 104-305) and 0 (IQ: 0-5).
3. 46% of patients assigned a placebo received at least one dose of darbepoetin as rescue therapy.
4. The placebo group received more intravenous iron (20.4 against 14.8%; p < 0.001). There were no differences in the proportion of patients who received oral iron (66.8 against 68.6%; p = 0.25).
5. The placebo group required more transfusions: 496 (24.5%) against 297 (14.8%); HR: darbepoetin against placebo: 0.56; (CI: 49-0.65); p < 0.001.
6. Diastolic blood pressure was higher in those treated with darbepoetin. Mean: 73mmHg (IQ: 67-78) against 71mmHg (65-77); p < 0.001.
SECONDARY EFFECTS
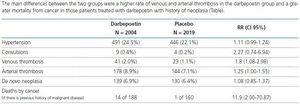
The main differences between the two groups were a higher rate of venous and arterial thrombosis in the darbepoetin group and a greater mortality from cancer in those patients treated with darbepoetin with history of neoplasia (Table 2).
¿ AUTHORS CONCLUSIONS
Treatment with darbepoetin alpha in patients with diabetes, with CKD and not on dialysis and with moderate anaemia does not reduce the combined risk of death and presentation of a cardiovascular event, or death and inclusion in renal replacement treatment, but is associated with an increased risk of ictus.
¿ REVIEWERS COMMENTS
The TREAT trial is a multicentre, randomised, double-blind study with rigorous methodology, that shows the absence of benefits from treatment with darbepoetin alpha, in terms of mortality, cardiovascular events and progression to end-stage kidney disease in the study population: diabetics with CKD and anaemia (Hb < 11g/dl) and with an elevated rate of cardiovascular morbidity and mortality (30% in 29 months of follow-up), which corresponds to that estimated in the calculation of sample size. It also casts serious doubts on the appropriateness of normalising Hb in this population since it increases the risk of ictus, thrombotic phenomena and, in those patients with history of neoplasias, the risk of mortality from cancer.
The TREAT trial confirms the findings of other non-blind studies such as the CREATE and the CHOIR. In these studies, erythropoiesis stimulating agents were used on CKD patients to obtain two treatment targets, Hb: 11 or 13-15g/dl and also showed no benefits in terms of lesser risk of mortality or cardiovascular events. Specifically, the CHOIR reported a greater cardiovascular morbidity and mortality in the high Hb group. In these two clinical trials, the highest Hb groups had received more iron supplements, suggesting that the treatment with iron could have been a confusing factor in the results. However, in the TREAT study, the group with the lowest Hb received more iron supplements, which would not support that hypothesis.
The benefits demonstrated in the TREAT study in the intervention group (lesser risk of receiving transfusions, lesser rate of coronary revascularisation and a slight increase in the score in one of the quality of life questionnaires), and the allogeneic sensitisation in potential renal transplant recipients because of the greater number of transfusions in the control group, compares unfavourably with the secondary effects described (greater risk of ictus and thrombotic phenomena, greater mortality from cancer if there is a history of neoplasias). This unfavourable safety profile coincides with the findings in other clinical trials in other populations, especially oncological.
However, some issues of the TREAT study should be taken into account. The first refers to the evolution of anaemia in the placebo group, with a median of 10.4g/dl and which increased to 10.6g/dl in the follow-up, implying that many patients in the placebo group would not have had anaemia secondary to treatment with erythropoiesis stimulating agents, in accordance with the current recommendations of the clinical practice guidelines. The study does not describe (it was not one of its objectives) whether the patients in the placebo group with persistently low haemoglobin, and which are those who, in clinical practice, suggest the appropriateness of intervention, had better or worse evolution than the treated group.
The second aspect is related to the dose of darbepoetin used in the intervention group, 176µg/month (IQ: 104-305), which indicates that many patients received higher doses than typically used in CKD patients not on dialysis. Observational studies and the subanalysis of studies like the CHOIR indicate that the higher the dose of epoetin required to obtain target Hb, the greater the risk of complications. Whether this tendency also applies to patients in the TREAT study, is not addressed and will probably be the focus of analysis in other studies.
¿ REVIEWERS COMMENTS
This study, along with the available evidence, shows that treatment with erythropoiesis stimulating factors in an attempt to normalise Hb levels in diabetic patients with CKD not on dialysis, does not provide benefits in terms of morbidity and mortality and raises doubts about the safety of this strategy.
Table 1. PRIMARY AND SECONDARY VARIABLES
Table 2. SECONDARY EFFECTS