Kidney involvement in mantle cell lymphoma is rare. We report a case of mantle cell lymphoma diagnosed after rapidly progressive glomerulonephritis with biopsy-proven paraneoplastic anti-neutrophil cytoplasmic antibody positive pauci-immune cresentic glomerulonephritis. Lymphoma treatment resulted in improved kidney function. This report demonstrates that pauci-immune cresentic glomerulonephritis can cause kidney impairment as the initial manifestation of mantle cell lymphoma. Physicians should be aware of the atypical presentations of the disease.
La afectación renal en el linfoma de células del manto es poco frecuente. Se presenta un caso de linfoma de células del manto diagnosticado tras una glomerulonefritis de progresión rápida con una glomerulonefritis paraneoplásica con anticuerpos anticitoplasma de neutrófilos positivos. El tratamiento del linfoma mejoró la función renal. Este informe demuestra que la glomerulonefritis pauciinmune puede causar insuficiencia renal como manifestación inicial del linfoma de células del manto. Los médicos deben conocer las presentaciones atípicas de la enfermedad.
Mantle cell lymphoma (MCL) is a rare and aggressive subtype of B-cell non-Hodgkin lymphoma (B-NHL) with diverse molecular and clinical characteristics, representing 3–10% of adult-onset B-NHL.1 Lymphoma can involve the kidneys through various mechanisms, including prerenal causes, obstruction of urine outflow, lymphomatous infiltration of kidneys, therapy-related consequences.2–4 However, unlike other lymphomas, kidney involvement of MCL has been rarely described.5 Here, we describe a rare case of MCL presented with anti-neutrophil cytoplasmic antibody (ANCA) positive pauci-immune glomerulonephritis (GN).
Case reportA 56-year-old male, with a history of diabetes mellitus and hypertension, presented with dark-colored, decreased urine output for 3 days. Antibiotics and supportive treatment were prescribed two weeks ago for the upper respiratory tract infection symptoms. He did not describe fever, night sweats, or weight loss (B symptoms) His family history was unremarkable. His vital signs showed temperature of 36.6°C, blood pressure of 155/80mmHg, pulse rate of 70beats/min, and oxygen saturation of 96% on room air. The patient had an ECOG Performance Status 0 and physical examination was remarkable for painless, fixed, and well-circumscribed lymphadenopathies in the bilateral cervical, axillary and inguinal regions with no hepatosplenomegaly. Laboratory evaluation revealed acute kidney injury (AKI) with serum creatinine (Scr) of 4.19mg/dL (0.72mg/dL 3 months prior). Initial laboratory tests were as follows: hemoglobin, 8.3g/dL; leukocytes, 10.97×109/L; platelets, 247×109/L; blood urea nitrogen, 63mg/dL; serum potassium 3.9mEq/L, and lactate dehydrogenase 203U/L. Urinalysis showed leucocytes 17/HPF, erythrocytes 355/HPF with many dysmorphic erythrocytes, and erythtocyte casts. Urine protein/creatinine ratio was 4228mg/g. Serological tests including hepatitis B, hepatitis C, HIV, anti-nuclear antibody and anti-glomerular basement membrane were all negative. The complement levels were within normal limits and no monoclonal bands were detected in serum and urine immunofixation. ANCA type proteinase 3 (PR3) was positive at 30.18U/mL (Normal range: 0–9.99U/mL). Abdomen ultrasound showed normal-sized kidneys without hydronephrosis. His Scr level rose to 9.28mg/dL during 3 days of admission and as the patient became anuric hemodialysis was started.
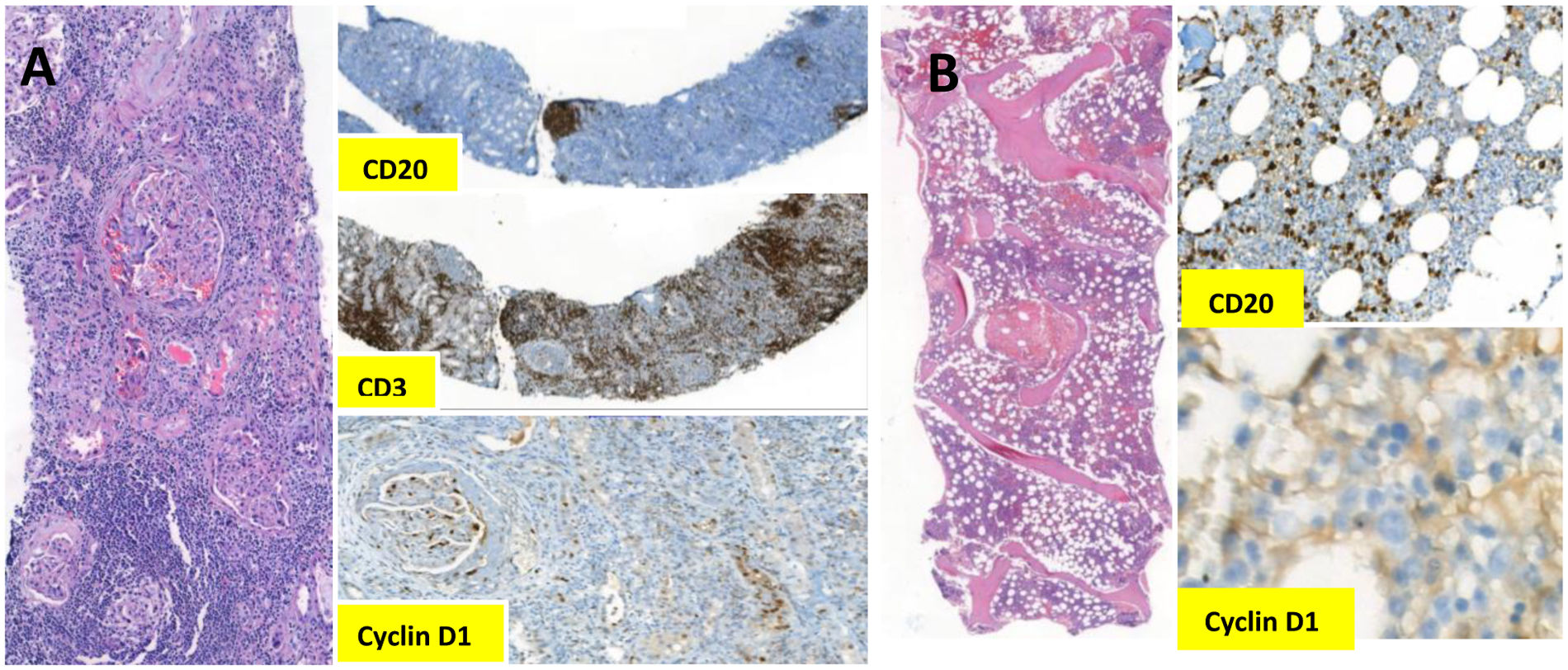
Kidney biopsy was performed on day 5 to evaluate the cause of rapidly progressive glomerulonephritis (RPGN). The biopsy included a total of 12 glomeruli, of which 7 showed cellular crescent formation and 2 had fibrinoid necrosis. There were severe multifocal tubulitis and interstitial inflammation rich in lymphocytes, plasma cells and eosinophils. Nodular arteriolar hyalinosis and severe arterial intimal fibrosis were observed in vascular structures. Interstitial lymphoid cells were negative for cyclin D1 and CD23 by immunohistochemistry. Immunofluorescence was positive for C3 in glomeruli. IgG, IgM, IgA, fibrinogen, C1q and light chains were negative (Fig. 1A). The findings supported diagnosis of pauci-immune cresentic GN and high-dose methylprednisolone was started.
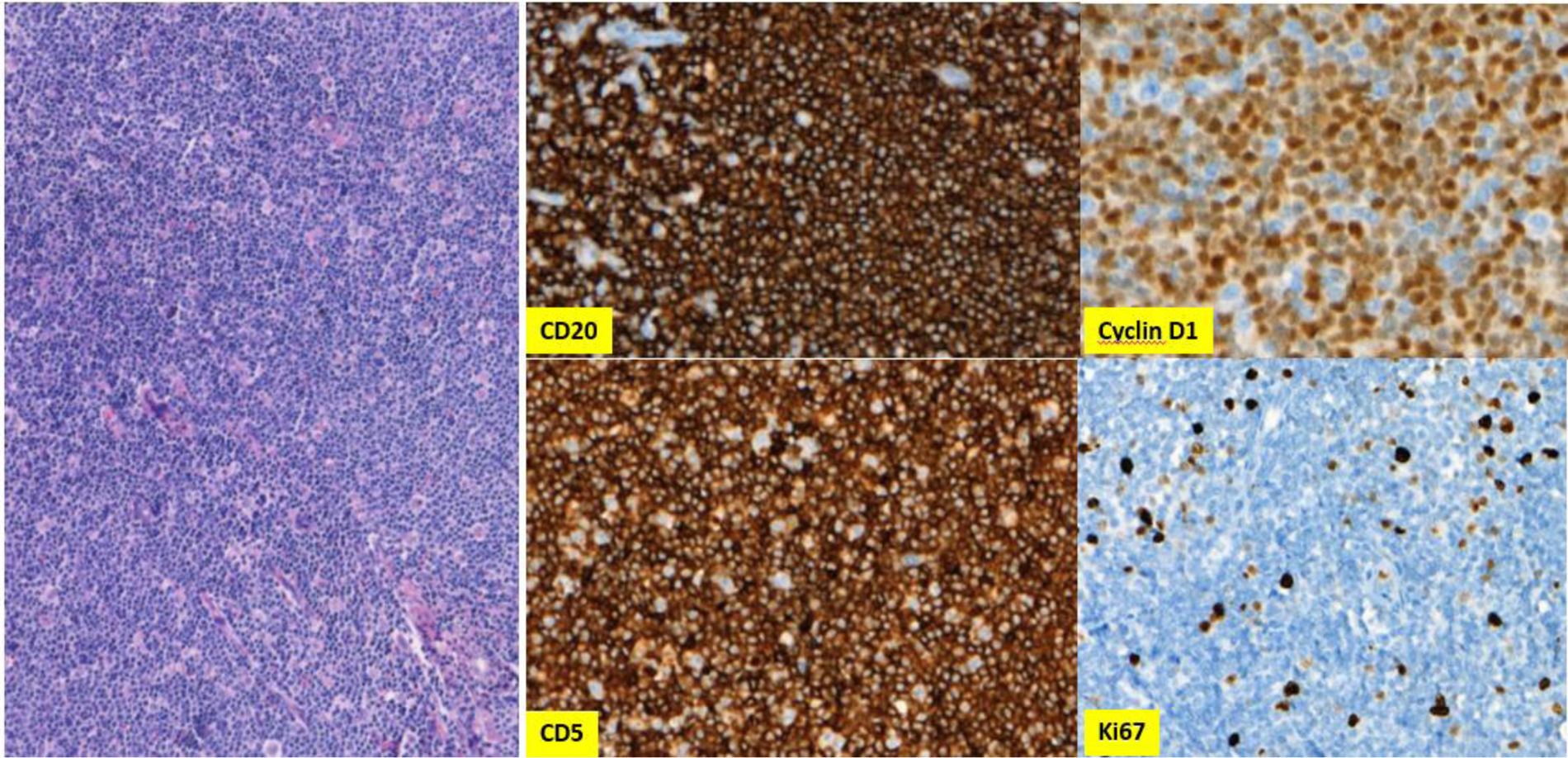
Meanwhile, 18F-fluorodeoxy glucose-positron emission tomography/computed tomogrophy (PET/CT) revealed hotspots in bilateral neck, axillae, mediastinum and abdominopelvic lymph nodes. Excisional inguinal lymph node biopsy was performed, which showed effaced architecture, predominantly nodular or, in some foci, diffusely infiltrated by monotonous small lymphoid cells. Immunohistochemistry revealed that these infiltrative cells were strongly positive for CD20, CD5, and cyclin D1, but negative for CD23 (Fig. 2). The Ki67 index was 10%. On multicolor flowcytometry, gated CD19 positive events (15% of viable cells) showed positivity for CD5, CD20, CD79b and surface lambda light chains. They were negative for CD10, CD23 and surface kappa light chains. These findings were suggestive for mantle cell lymphoma, confirmed by the detection of translocation t(11;14) by FISH (fluorescence in situ hybridization). Subsequent bone marrow biopsy showed minimal bone marrow involvement by lymphoma (Fig. 1B).
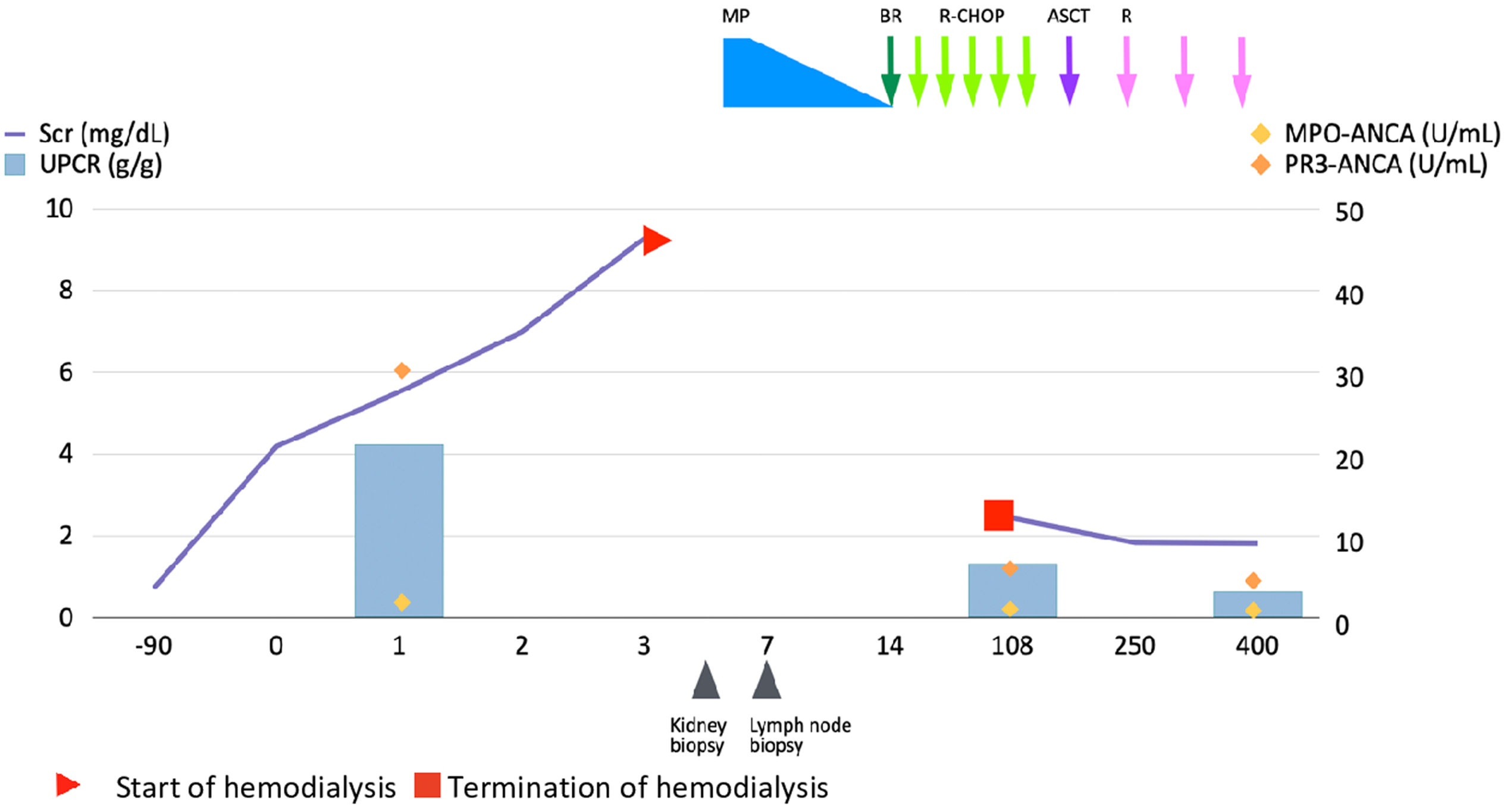
Altogether, a final diagnosis of stage IVA with a high-risk mantle cell lymphoma with pauci-immune GN was made. International Prognostic Index-C (MIPI-C) score was 6.1, stratified patient to intermediate risk. The patient was immediately started on R-CHOP (rituximab, cyclophosphamide, vincristine, doxorubicin and prednisolone) chemotherapy and discharged on maintenance hemodialysis. After 3 cycles of chemotherapy the axillary, hilar and abdominopelvic lymph nodes decreased in size. Furthermore, PET/CT confirmed reduced metabolic activities. His kidney function improved and hemodialysis discontinued after 12 weeks of therapy. Complete response was achieved after 6 cycles of R-CHOP chemotherapy and autologous stem cell transplantation (ASCT) was performed. After the ASCT, he was initiated Rituximab maintenance every two months, which will be complemented in 2 years. Fig. 3 shows clinical course of the patient.
Clinical course. After initiating the treatment, patient's kidney function improved and hemodialysis discontinued. Serum titers of PR3-ANCA normalized. His follow-up continues with lymphoma remission and chronic kidney disease stage G3bA3. ANCA anti-neutrophil cytoplasmic antibody; ASCT autologous stem cell transplantation; BR bendamustine, rituximab; MP methylprednisolone; MPO myeloperoxidase; PR3 proteinase-3; R rituximab; R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone; Scr serum creatinine; UPCR urinary protein creatinine ratio.
Paraneoplastic GNs, though rare, is a well-recognized complication associated with lymphomas.6,7 Possible mechanisms to explain the pathophysiology include altered immune response, immune complex deposition, tumor antigens and viral antigens. A wide spectrum of glomerular lesions can be seen depending on the underlying lymphoproliferative disorder.3,4,8 Minimal change disease (MCD) is predominantly observed in conjunction with Hodgkin lymphoma, while membranoproliferative GN and membranous nephropathy are more frequently associated with NHL. In a series of 66 NHL patients with manifestations of kidney failure, GN was identified in only 4 cases.3 Given that MCL is a rare subtype of B-NHL, kidney involvement is even less frequently reported. Several case reports have documented MCL with direct kidney infiltration and various paraneoplastic GN subtypes, including proliferative GN, membranoproliferative GN, MCD, focal segmental glomerulosclerosis, immune complex-mediated GN, and crescentic GN.5,9–15
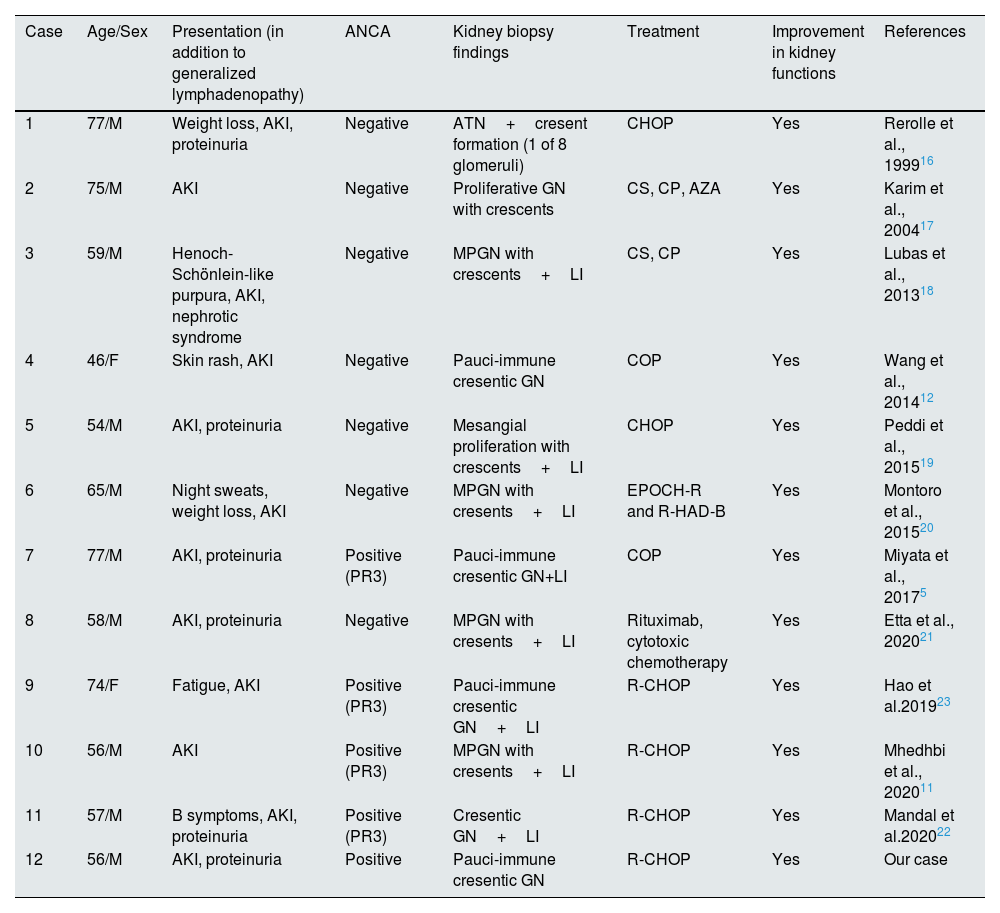
To date, 11 cases of GN with crescent formation associated with MCL have been reported (Table 1).5,11,12,16–23 All cases presented with multiple lymphadenopathies and AKI, with kidney recovery observed following chemotherapy regimen for MCL. Notably, only 4 cases were ANCA positive.5,11,22,23 To our knowledge, our patient is the third reported case of MCL with ANCA positive pauci-immune GN. Miyata et al. reported a 77-year-old man who presented with complaints of fatigue and decreased appetite for several months, along with AKI and proteinuria.5 Kidney biopsy revealed MCL infiltration and pauci-immune cresentic GN, accompanied by serum PR3-ANCA positivity. The second reported case was a 74-year-old female who was also diagnosed with both MCL and ANCA-associated vasculitis.23 The kidney functions of both patients improved after chemotherapy, similar to our patient. Our case differ from these with no tubulointerstitial infiltration by malignant lymphoma cells.
Glomerulonephritis with cresent formation associated with mantle cell lymphoma in previous case reports.
| Case | Age/Sex | Presentation (in addition to generalized lymphadenopathy) | ANCA | Kidney biopsy findings | Treatment | Improvement in kidney functions | References |
|---|---|---|---|---|---|---|---|
| 1 | 77/M | Weight loss, AKI, proteinuria | Negative | ATN+cresent formation (1 of 8 glomeruli) | CHOP | Yes | Rerolle et al., 199916 |
| 2 | 75/M | AKI | Negative | Proliferative GN with crescents | CS, CP, AZA | Yes | Karim et al., 200417 |
| 3 | 59/M | Henoch-Schönlein-like purpura, AKI, nephrotic syndrome | Negative | MPGN with crescents+LI | CS, CP | Yes | Lubas et al., 201318 |
| 4 | 46/F | Skin rash, AKI | Negative | Pauci-immune cresentic GN | COP | Yes | Wang et al., 201412 |
| 5 | 54/M | AKI, proteinuria | Negative | Mesangial proliferation with crescents+LI | CHOP | Yes | Peddi et al., 201519 |
| 6 | 65/M | Night sweats, weight loss, AKI | Negative | MPGN with cresents+LI | EPOCH-R and R-HAD-B | Yes | Montoro et al., 201520 |
| 7 | 77/M | AKI, proteinuria | Positive (PR3) | Pauci-immune cresentic GN+LI | COP | Yes | Miyata et al., 20175 |
| 8 | 58/M | AKI, proteinuria | Negative | MPGN with cresents+LI | Rituximab, cytotoxic chemotherapy | Yes | Etta et al., 202021 |
| 9 | 74/F | Fatigue, AKI | Positive (PR3) | Pauci-immune cresentic GN+LI | R-CHOP | Yes | Hao et al.201923 |
| 10 | 56/M | AKI | Positive (PR3) | MPGN with cresents+LI | R-CHOP | Yes | Mhedhbi et al., 202011 |
| 11 | 57/M | B symptoms, AKI, proteinuria | Positive (PR3) | Cresentic GN+LI | R-CHOP | Yes | Mandal et al.202022 |
| 12 | 56/M | AKI, proteinuria | Positive | Pauci-immune cresentic GN | R-CHOP | Yes | Our case |
AKI acute kidney injury; ANCA anti-neutrophil cytoplasmic antibody; ATN acute tubular necrosis; AZA azathioprine; CHOP cyclophosphamide, vincristine, doxorubicin, prednisolone; COP cyclophosphamide, vincristine, prednisolone; CP cyclophosphamide; CS corticosteroids; EPPCH-R rituximab, etoposide, prednisone, vincristine, adriamycin, cyclophosphamide; F female; GN glomerulonephritis; LI lymphomatous infiltration; MPGN membranoproliferative glomerulonephritis; M male; R-HAD-B rituximab, bortezomid, citarabine, dexamethasone.
ANCA positivity is very rare in lymphoid malignancies.8,24 Moreover, no correlation has been established between ANCA positivity and the presence of rheumatic symptoms or vasculitis. The pathogenetic mechanisms through which neoplasms induce ANCA-associated vasculitis and RPGN remain largely unknown. There are hypotheses suggesting that altered PR3 expression and function, as well as dysregulation of the T-cell immune response, may be underlying causes.6,25 Despite the absence of immune deposits, pauci-immune crescentic GN is an immune-mediated disease and studies implicate the role of ANCA in its pathogenesis. The specific contribution of pr3-ANCA in the development of paraneoplastic GN warrants further investigation.
Kidney involvement may manifest as an initial indicator of MCL or subsequently following its diagnosis. Clinical features commonly associated with lymphoma, such as fever, lymphadenopathy, night sweats, skin involvement, and weight loss, can also be observed in ANCA-associated vasculitis (Table 1). Therefore, a kidney biopsy is essential to determine the cause of kidney damage and prevent missing the diagnosis of a potentially life-threating condition such as ANCA-associated vasculitis. This approach may also facilitate the initial diagnosis of lymphoma, as in our patient. Although the diagnose of ANCA-associated vasculitis does not alter the initial treatment approach, which is a part of standart MCL treatment, it is crucial for determining the prognosis and follow-up strategy for these patients. It is noteworthy that the remission of lymphoma has been observed to coincide with the remission of glomerulopathy, as well as with relapses. Therefore, recurrence of glomerulonephritis or an elevation in PR3-ANCA titer may serve as indicators of lymphoma recurrence.
Our case underscores the importance of considering the association between lymphoma and ANCA-positive pauci-immune GN in the differential diagnosis for kidney impairment, highlighting that early diagnosis and treatment can significantly improve kidney and patient outcomes.
Authors’ contributionsGK is first author: study concept, data collection, data analysis and interpretation, writing and revision. MY, SK: study design, data analysis, interpretation and revision. YG, ESY, IK, ÖA: study design, and revision. All authors have read and approved the manuscript.
Consent for publicationThe patient signed an informed consent form and agreed with any scientific publication.
EthicsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
FundingThe authors declared that this study has received no financial support.
Conflict of interestThe authors have no conflict of interest to declare.
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.