Kidney transplant (KT) recipients who experience graft failure and return to dialysis face a higher risk of adverse outcomes. This study aimed to identify risk factors for hospitalization and mortality two years post-graft failure.
Materials and methodsWe conducted a retrospective cohort study of end-stage kidney disease patients who initiated hemodialysis following graft failure between January 2019 and December 2020. The Clinical Frailty Scale (CFS) and the Charlson Comorbidity Index (CCI) were assessed for each patient at the time of graft loss. The primary outcomes were hospitalization and all-cause mortality over a two-year follow-up period.
ResultsA total of 107 patients were included, with a mean age of 55.9 years and a mean graft survival of 134 months. The two-year hospitalization rate was 37.4%, with lower residual diuresis and higher CFS identified as independent risk factors. The two-year mortality rate was 16.8%. A multivariate regression model, explaining 82% of the variance, confirmed that higher CCI, higher CFS, and lower residual diuresis significantly increased mortality risk. A CCI cut-off of ≥8 (AUC 0.95) further stratified patients at elevated mortality risk. Immunological and transplant-related variables did not influence mortality or hospitalization risk.
ConclusionsIn this cohort, frailty defined by CFS was associated with hospitalization and mortality, while comorbidity burden evaluated by CCI was strongly related to mortality. These tools may help personalize the care of patients with a failing graft.
Los receptores de trasplante renal (TR) que experimentan pérdida del injerto y requieren reiniciar la diálisis presentan un mayor riesgo de eventos adversos. Este estudio tuvo como objetivo identificar predictores de hospitalización y mortalidad durante los dos años posteriores a la pérdida del injerto.
Materiales y métodosSe realizó un estudio de cohorte retrospectivo en receptores de TR que iniciaron hemodiálisis tras la pérdida del injerto entre enero de 2019 y diciembre de 2020. La fragilidad clínica en el momento de la pérdida del injerto se evaluó mediante la Clinical Frailty Scale (CFS), y la carga de comorbilidad se valoró con el Índice de Comorbilidad de Charlson (CCI). Los desenlaces primarios fueron la hospitalización y la mortalidad por cualquier causa durante un seguimiento de dos años.
ResultadosSe incluyeron 107 pacientes, con una edad media de 55.9 años y una mediana de supervivencia del injerto de 134 meses. La tasa de hospitalización a los dos años fue del 37,4%, identificándose una menor diuresis residual y un CFS elevado como factores de riesgo independientes. La tasa de mortalidad fue del 16,8%. Un modelo de regresión multivariable (R2 = 0,82) mostró que un CCI y CFS más elevados, junto con una diuresis residual reducida, se asociaban significativamente con un mayor riesgo de mortalidad. Un punto de corte de CCI ≥ 8 (AUC = 0,95) permitió estratificar a los pacientes con mayor riesgo. No se observó asociación entre variables inmunológicas o relacionadas con el trasplante y los desenlaces clínicos.
ConclusionesEn esta cohorte, la fragilidad clínica evaluada mediante la CFS fue un predictor clave de hospitalización y mortalidad, mientras que una mayor carga de comorbilidad (CCI) se asoció significativamente con la mortalidad. La utilización de estas herramientas podría optimizar la estratificación del riesgo y facilitar un enfoque más personalizado en el manejo de pacientes con disfunción del injerto.
The last decade has witnessed a significant improvement in short-term kidney transplantation outcomes, but long-term graft survival remains suboptimal, with the mean graft survival rate at 10 years ranging from 50% to 70%.1,2 Additionally, the increasing patient lifespan and better access to healthcare have led to a growing population of kidney transplant (KT) recipients who experience graft failure and return to dialysis, which is currently the fourth leading cause of incident dialysis.3,4
Patients with end-stage kidney disease (ESKD) who begin dialysis after graft loss encounter various complications and face an increased burden of morbidity beyond chronic kidney disease (CKD). Previous studies have indicated lower quality of life scores, a higher incidence of depression, increased hospitalizations, and notably higher mortality rates.5–8 Certain characteristics of this patient group may contribute to the poorer outcomes observed, including prolonged CKD duration and ongoing exposure to immunosuppression (IS), which is linked to an increased risk of life-threatening infections,9 endocrine and metabolic dysfunction,10 and specific types of neoplasms.11
Considering the aging global population and the improved survival rates of KT recipients, we are witnessing a growing number of older, frailer patients with more comorbidities returning to dialysis, which might also influence the increased morbidity and mortality rates post-graft failure.12
The Clinical Frailty Scale (CFS) and the Charlson Comorbidity Index (CCI) have been utilized to assess frailty and clinical outcomes in patients with ESKD, including dialysis, KT recipients, and those under conservative care management.13–17 Frailty, a multisystem clinical syndrome resulting from the accumulation of vascular, inflammatory, and age-related challenges, is increasingly prevalent in the CKD hemodialysis (HD) population and significantly impacts clinical outcomes such as hospitalization, institutionalization, decreased quality of life, and mortality.18 The CCI score serves as a valid index with strong discriminatory power in predicting survival at the onset of dialysis.17 For patients facing high morbidity and mortality rates following graft loss, these indices offer valuable tools for multidisciplinary decision-making regarding whether to initiate dialysis or pursue conservative kidney management, engaging both patients and their families.16,19
The community of physicians involved in caring for KT recipients is increasingly aware of the importance of managing post-graft loss.20 Still, coordination with dialysis nephrologists, conservative care teams, and timely referrals for retransplantation remains suboptimal.21 Despite the high morbidity and mortality observed in this patient population, the systematic assessment of frailty and comorbidities is still scarce. Consequently, the potential role of these factors in guiding therapeutic decisions and informing prognosis has been underexplored.22,23
The primary aim of our study was to identify predictors of hospitalization and mortality over a two-year period among ESKD with a failed graft who initiated HD, focusing on comorbidity and frailty scores.
MethodsWe performed an observational retrospective cohort study of ESKD patients with graft failure who started HD between January 2019 and December 2020 at Coimbra University Hospital, a large tertiary care centre in Portugal. Our study was carried out following the Declaration of Helsinki and approved by the ethics committee of Coimbra University Hospital (#CE-002-2020). Given the study's retrospective nature, sample size, and outcome of our sample population, the same committee waived the need for informed consent.
Patients over 18 years old who had at least two appointments with a transplant nephrologist the year before HD initiation were included. Patients with a previous graft, multiorgan transplant, or graft failure within the first month post-transplant were excluded. Demographic data, comorbidities, and transplant-related variables, including biopsy-proven rejections according to Banff 2017 criteria, were collected from patient records. We have also accessed patients IS withdrawal scheme post-graft failure. Laboratorial values, including serum albumin, hemoglobin and parathormone levels were registered in the last appointment post-graft failure.
The estimated glomerular filtration rate (eGFR) at graft failure was defined by the CKD-EPI formula. Whether planned or unplanned, the decision on start dialysis was based on clinical and laboratory parameters. An urgent HD start was defined as the need to begin HD imminently or within 48h after presentation to the Emergency Room. An unplanned start was defined as the absence of autologous vascular access and/or the requirement for hospitalization to start HD. Residual diuresis was defined as the 24-h urinary output (mL/day) at the last nephrology appointment.
Comorbidities, frailty, and cardiovascular risk assessmentTo classify patients’ cumulative comorbidities and frailty risk, we utilized the 2014 revised CCI24,25 and the CFS26 (supplementary Tables 1 and 2). To determine CCI and CFS, we relied on the information provided by the assistant nephrologist during the last nephrology appointment before graft failure, with a mean time to graft failure of 23±14 days.
For baseline cardiovascular (CV) risk, we referenced the European Society of Cardiology (ESC) guidelines.27 Because our focus was solely on ESKD patients, who are classified as very high risk for CVD, we further stratified CV risk irrespective of kidney disease. Based on the ESC guidelines, we divided CV risk categories into High (ESC's very high and high risk) and Low (ESC's moderate and low risk). All patients had at least one echocardiogram in the year before graft failure, as it is part of our KT follow-up routine, which allowed us to classify patients as having heart failure with or without preserved ejection fraction.
Follow-up and primary endpointsWe followed our cohort for two years, and the primary endpoints were all-cause hospitalization and mortality. Hospitalization was defined as an admission to our inpatient facility for medical observation, treatment, or care, with a minimum stay of 24h. We registered the number of hospitalizations by patient, cause, and duration. The cause of hospitalization was classified as infectious, CV, graft intolerance syndrome or neoplasia, based on the primary reason for admission documented in the medical records. For mortality, the cause of death was established according to the patient's registries and classified as infectious cause, CV events, neoplasms, and others.
Statistical analysisStatistical analysis was performed using IBM-SPSS Statistics v22, and the confidence interval (CI) was set at 95%. A p-value <0.05 was considered statistically significant. Categorical variables were described as relative frequency (absolute frequency). Continuous data (including the CCI and CFS) were defined as mean±standard deviation. T-tests were used to compare the means of normally distributed variables between groups, and the χ2 test was used to compare the prevalence of categorical variables of interest. A comparison of continuous variables is presented as Pearson coefficient (r). Logistic regression analysis was used to evaluate hospitalization risk, and Cox regression analysis was used to assess mortality risk factors. The variables considered in the multivariate models were those statistically significant in univariate analysis and clinically relevant variables. The receiver operating characteristic (ROC) curves and Youden index were used to identify a cut-off point for variables of interest that predicted higher mortality.
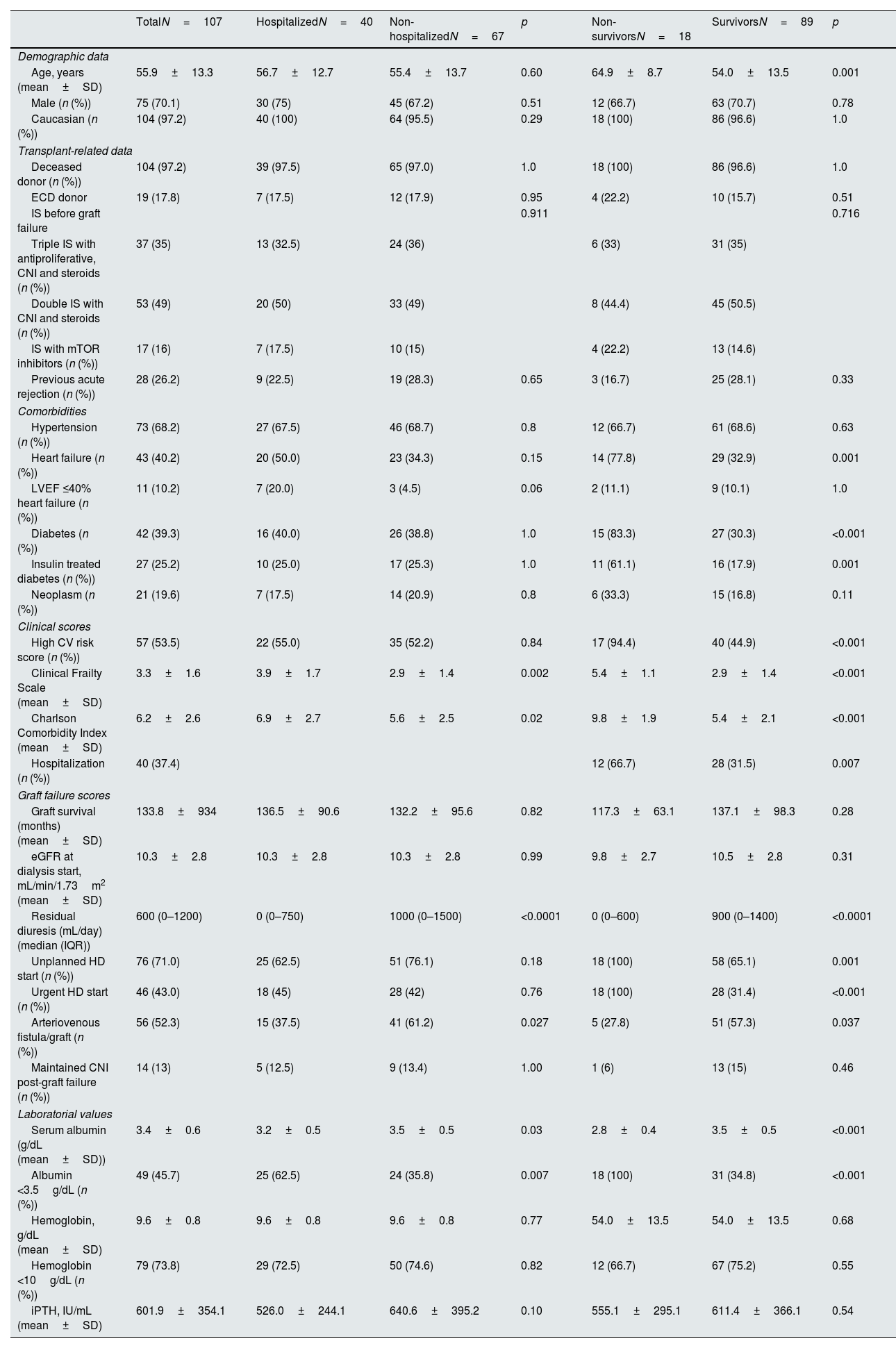
ResultsDemographic data and baseline characteristics at the start of hemodialysisA total of 107 patients met the inclusion and exclusion criteria and were included, with their demographic data summarized in Table 1. There was a male predominance, and the mean age at graft loss was 55.9±13.3 years. The IS therapy before HD initiation varied: fifty-three patients (49%) were on double therapy with steroids and calcineurin inhibitors (CNI); 37 patients (35%) were on triple IS with CNI, mycophenolate, and steroids; and 17 patients (16%) had an IS regimen with mTOR inhibitors. After graft loss, all patients on a triple IS regimen suspended mycophenolate immediately. Regarding CNI, 14 patients who were candidates for retransplantation and were not HLA sensitized at graft loss maintained calcineurin inhibitors for six months in addition to prednisolone. All patients maintained 5mg prednisolone for at least one year. There was a high prevalence of significant CV comorbidities such as hypertension, heart failure, and diabetes, with an average duration of diabetes of 11.5±6.7 years. This is reflected in more than half of the patients presenting with a high ESC CV score. The mean graft survival time was approximately 11 years (133.8±93.4 months), and the leading cause of graft loss was presumed chronic allograft nephropathy. The majority of patients (N=76, 71%) had an unplanned HD start, and of these, a large proportion (N=46 patients, 60.5%) had urgent initiation, mainly due to hypervolemia (N=41, 90%).
Demographic, clinical, and laboratory characteristics of kidney transplant recipients with graft loss.
| TotalN=107 | HospitalizedN=40 | Non-hospitalizedN=67 | p | Non-survivorsN=18 | SurvivorsN=89 | p | |
|---|---|---|---|---|---|---|---|
| Demographic data | |||||||
| Age, years (mean±SD) | 55.9±13.3 | 56.7±12.7 | 55.4±13.7 | 0.60 | 64.9±8.7 | 54.0±13.5 | 0.001 |
| Male (n (%)) | 75 (70.1) | 30 (75) | 45 (67.2) | 0.51 | 12 (66.7) | 63 (70.7) | 0.78 |
| Caucasian (n (%)) | 104 (97.2) | 40 (100) | 64 (95.5) | 0.29 | 18 (100) | 86 (96.6) | 1.0 |
| Transplant-related data | |||||||
| Deceased donor (n (%)) | 104 (97.2) | 39 (97.5) | 65 (97.0) | 1.0 | 18 (100) | 86 (96.6) | 1.0 |
| ECD donor | 19 (17.8) | 7 (17.5) | 12 (17.9) | 0.95 | 4 (22.2) | 10 (15.7) | 0.51 |
| IS before graft failure | 0.911 | 0.716 | |||||
| Triple IS with antiproliferative, CNI and steroids (n (%)) | 37 (35) | 13 (32.5) | 24 (36) | 6 (33) | 31 (35) | ||
| Double IS with CNI and steroids (n (%)) | 53 (49) | 20 (50) | 33 (49) | 8 (44.4) | 45 (50.5) | ||
| IS with mTOR inhibitors (n (%)) | 17 (16) | 7 (17.5) | 10 (15) | 4 (22.2) | 13 (14.6) | ||
| Previous acute rejection (n (%)) | 28 (26.2) | 9 (22.5) | 19 (28.3) | 0.65 | 3 (16.7) | 25 (28.1) | 0.33 |
| Comorbidities | |||||||
| Hypertension (n (%)) | 73 (68.2) | 27 (67.5) | 46 (68.7) | 0.8 | 12 (66.7) | 61 (68.6) | 0.63 |
| Heart failure (n (%)) | 43 (40.2) | 20 (50.0) | 23 (34.3) | 0.15 | 14 (77.8) | 29 (32.9) | 0.001 |
| LVEF ≤40% heart failure (n (%)) | 11 (10.2) | 7 (20.0) | 3 (4.5) | 0.06 | 2 (11.1) | 9 (10.1) | 1.0 |
| Diabetes (n (%)) | 42 (39.3) | 16 (40.0) | 26 (38.8) | 1.0 | 15 (83.3) | 27 (30.3) | <0.001 |
| Insulin treated diabetes (n (%)) | 27 (25.2) | 10 (25.0) | 17 (25.3) | 1.0 | 11 (61.1) | 16 (17.9) | 0.001 |
| Neoplasm (n (%)) | 21 (19.6) | 7 (17.5) | 14 (20.9) | 0.8 | 6 (33.3) | 15 (16.8) | 0.11 |
| Clinical scores | |||||||
| High CV risk score (n (%)) | 57 (53.5) | 22 (55.0) | 35 (52.2) | 0.84 | 17 (94.4) | 40 (44.9) | <0.001 |
| Clinical Frailty Scale (mean±SD) | 3.3±1.6 | 3.9±1.7 | 2.9±1.4 | 0.002 | 5.4±1.1 | 2.9±1.4 | <0.001 |
| Charlson Comorbidity Index (mean±SD) | 6.2±2.6 | 6.9±2.7 | 5.6±2.5 | 0.02 | 9.8±1.9 | 5.4±2.1 | <0.001 |
| Hospitalization (n (%)) | 40 (37.4) | 12 (66.7) | 28 (31.5) | 0.007 | |||
| Graft failure scores | |||||||
| Graft survival (months) (mean±SD) | 133.8±934 | 136.5±90.6 | 132.2±95.6 | 0.82 | 117.3±63.1 | 137.1±98.3 | 0.28 |
| eGFR at dialysis start, mL/min/1.73m2 (mean±SD) | 10.3±2.8 | 10.3±2.8 | 10.3±2.8 | 0.99 | 9.8±2.7 | 10.5±2.8 | 0.31 |
| Residual diuresis (mL/day) (median (IQR)) | 600 (0–1200) | 0 (0–750) | 1000 (0–1500) | <0.0001 | 0 (0–600) | 900 (0–1400) | <0.0001 |
| Unplanned HD start (n (%)) | 76 (71.0) | 25 (62.5) | 51 (76.1) | 0.18 | 18 (100) | 58 (65.1) | 0.001 |
| Urgent HD start (n (%)) | 46 (43.0) | 18 (45) | 28 (42) | 0.76 | 18 (100) | 28 (31.4) | <0.001 |
| Arteriovenous fistula/graft (n (%)) | 56 (52.3) | 15 (37.5) | 41 (61.2) | 0.027 | 5 (27.8) | 51 (57.3) | 0.037 |
| Maintained CNI post-graft failure (n (%)) | 14 (13) | 5 (12.5) | 9 (13.4) | 1.00 | 1 (6) | 13 (15) | 0.46 |
| Laboratorial values | |||||||
| Serum albumin (g/dL (mean±SD)) | 3.4±0.6 | 3.2±0.5 | 3.5±0.5 | 0.03 | 2.8±0.4 | 3.5±0.5 | <0.001 |
| Albumin <3.5g/dL (n (%)) | 49 (45.7) | 25 (62.5) | 24 (35.8) | 0.007 | 18 (100) | 31 (34.8) | <0.001 |
| Hemoglobin, g/dL (mean±SD) | 9.6±0.8 | 9.6±0.8 | 9.6±0.8 | 0.77 | 54.0±13.5 | 54.0±13.5 | 0.68 |
| Hemoglobin <10g/dL (n (%)) | 79 (73.8) | 29 (72.5) | 50 (74.6) | 0.82 | 12 (66.7) | 67 (75.2) | 0.55 |
| iPTH, IU/mL (mean±SD) | 601.9±354.1 | 526.0±244.1 | 640.6±395.2 | 0.10 | 555.1±295.1 | 611.4±366.1 | 0.54 |
SD: standard deviation; ECD: expanded criteria donor; IS: immunosuppression; CNI: calcineurin inhibitors; mTOR: mammalian target of rapamycin inhibitors; LVEF: left ventricular ejection fraction; CV: cardiovascular; eGFR: estimated glomerular filtration rate; HD: hemodialysis; iPTH: intact parathormone.
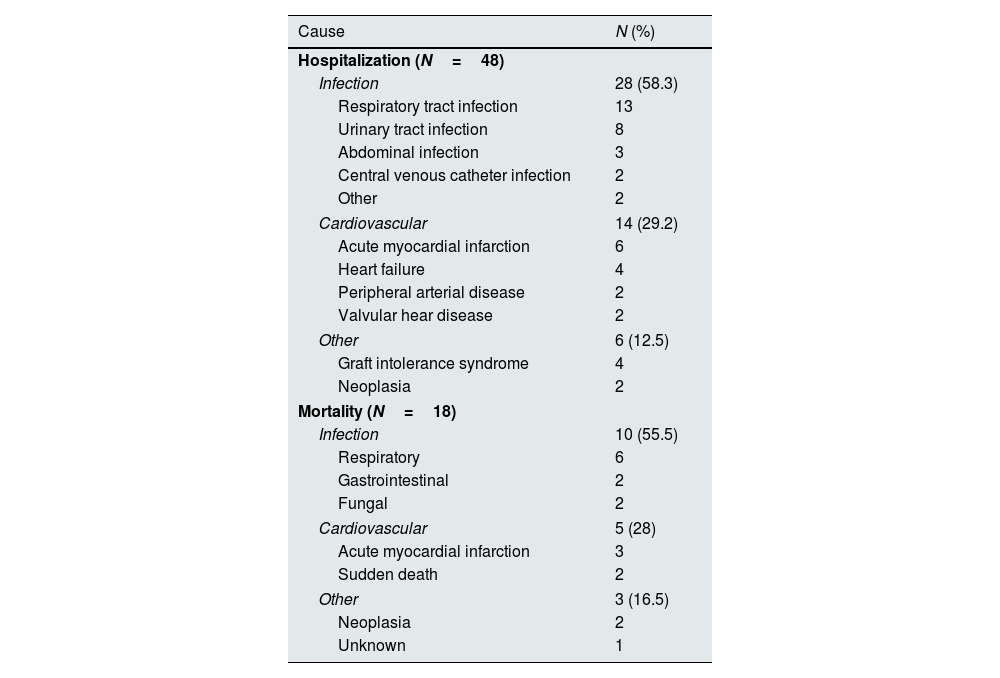
We recorded 48 hospitalizations (0.44 events per patient) and a hospitalization rate of 37.4% (n=40) at two years of follow-up, with a median hospitalization stay of 17.6 days (IQR 1–22). The most common causes of hospitalization were infections (58.3%, n=28), especially respiratory and urinary tract infections, followed by CV events (29%, n=14), and four patients were hospitalized due to graft intolerance syndrome (Table 2).
Causes of hospitalization and death.
| Cause | N (%) |
|---|---|
| Hospitalization (N=48) | |
| Infection | 28 (58.3) |
| Respiratory tract infection | 13 |
| Urinary tract infection | 8 |
| Abdominal infection | 3 |
| Central venous catheter infection | 2 |
| Other | 2 |
| Cardiovascular | 14 (29.2) |
| Acute myocardial infarction | 6 |
| Heart failure | 4 |
| Peripheral arterial disease | 2 |
| Valvular hear disease | 2 |
| Other | 6 (12.5) |
| Graft intolerance syndrome | 4 |
| Neoplasia | 2 |
| Mortality (N=18) | |
| Infection | 10 (55.5) |
| Respiratory | 6 |
| Gastrointestinal | 2 |
| Fungal | 2 |
| Cardiovascular | 5 (28) |
| Acute myocardial infarction | 3 |
| Sudden death | 2 |
| Other | 3 (16.5) |
| Neoplasia | 2 |
| Unknown | 1 |
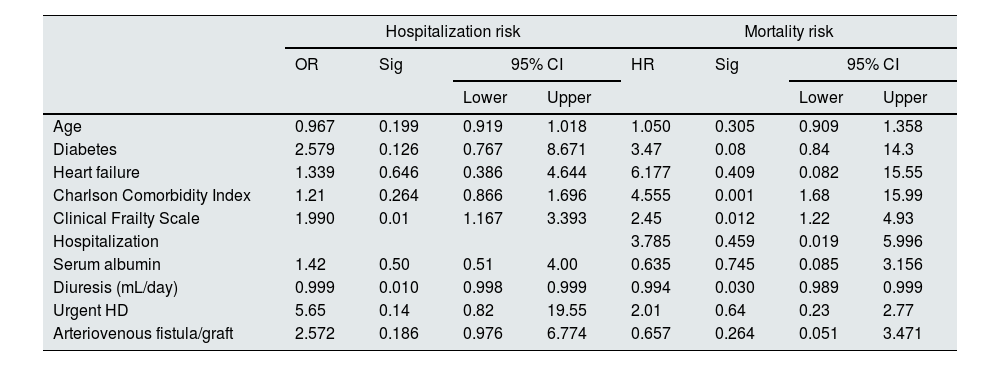
Table 1 presents patients’ clinical and laboratory features with and without hospitalizations. Identified risk factors for hospitalization included lower diuresis, lower albumin levels, the presence of a central venous catheter (CVC), higher CFS, and higher CCI scores. Age, gender, baseline CV risk, specific comorbidities (neoplasms, diabetes, heart failure), transplant-related, and immunological variables did not influence the hospitalization rate at two years. Our multivariate model (Table 3) explained 39.2% of the sample's variance and showed that residual diuresis volume and CFS at graft loss were the only significant parameters increasing hospitalization risk. Residual diuresis was correlated with CV hospitalizations, as these patients had a significantly lower residual diuresis volume (mean difference (df)=−423.1mL/day, p=0.02). Patients with unplanned HD initiation (df=7.7 days, p=0.0004), higher CFS (r=0.345, p=0.002), and higher CCI (r=0.257, p=0.03) had significantly more prolonged hospitalizations.
Multivariate regression analysis of hospitalization and mortality risk.
| Hospitalization risk | Mortality risk | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | Sig | 95% CI | HR | Sig | 95% CI | |||
| Lower | Upper | Lower | Upper | |||||
| Age | 0.967 | 0.199 | 0.919 | 1.018 | 1.050 | 0.305 | 0.909 | 1.358 |
| Diabetes | 2.579 | 0.126 | 0.767 | 8.671 | 3.47 | 0.08 | 0.84 | 14.3 |
| Heart failure | 1.339 | 0.646 | 0.386 | 4.644 | 6.177 | 0.409 | 0.082 | 15.55 |
| Charlson Comorbidity Index | 1.21 | 0.264 | 0.866 | 1.696 | 4.555 | 0.001 | 1.68 | 15.99 |
| Clinical Frailty Scale | 1.990 | 0.01 | 1.167 | 3.393 | 2.45 | 0.012 | 1.22 | 4.93 |
| Hospitalization | 3.785 | 0.459 | 0.019 | 5.996 | ||||
| Serum albumin | 1.42 | 0.50 | 0.51 | 4.00 | 0.635 | 0.745 | 0.085 | 3.156 |
| Diuresis (mL/day) | 0.999 | 0.010 | 0.998 | 0.999 | 0.994 | 0.030 | 0.989 | 0.999 |
| Urgent HD | 5.65 | 0.14 | 0.82 | 19.55 | 2.01 | 0.64 | 0.23 | 2.77 |
| Arteriovenous fistula/graft | 2.572 | 0.186 | 0.976 | 6.774 | 0.657 | 0.264 | 0.051 | 3.471 |
OR: odds ratio; HR: hazard ratio; Sig: significance; CI: confidence interval; HD: hemodialysis. Age, Charlson Comorbidity Index, Clinical Frailty Score, and diuresis were treated as continuous variables.
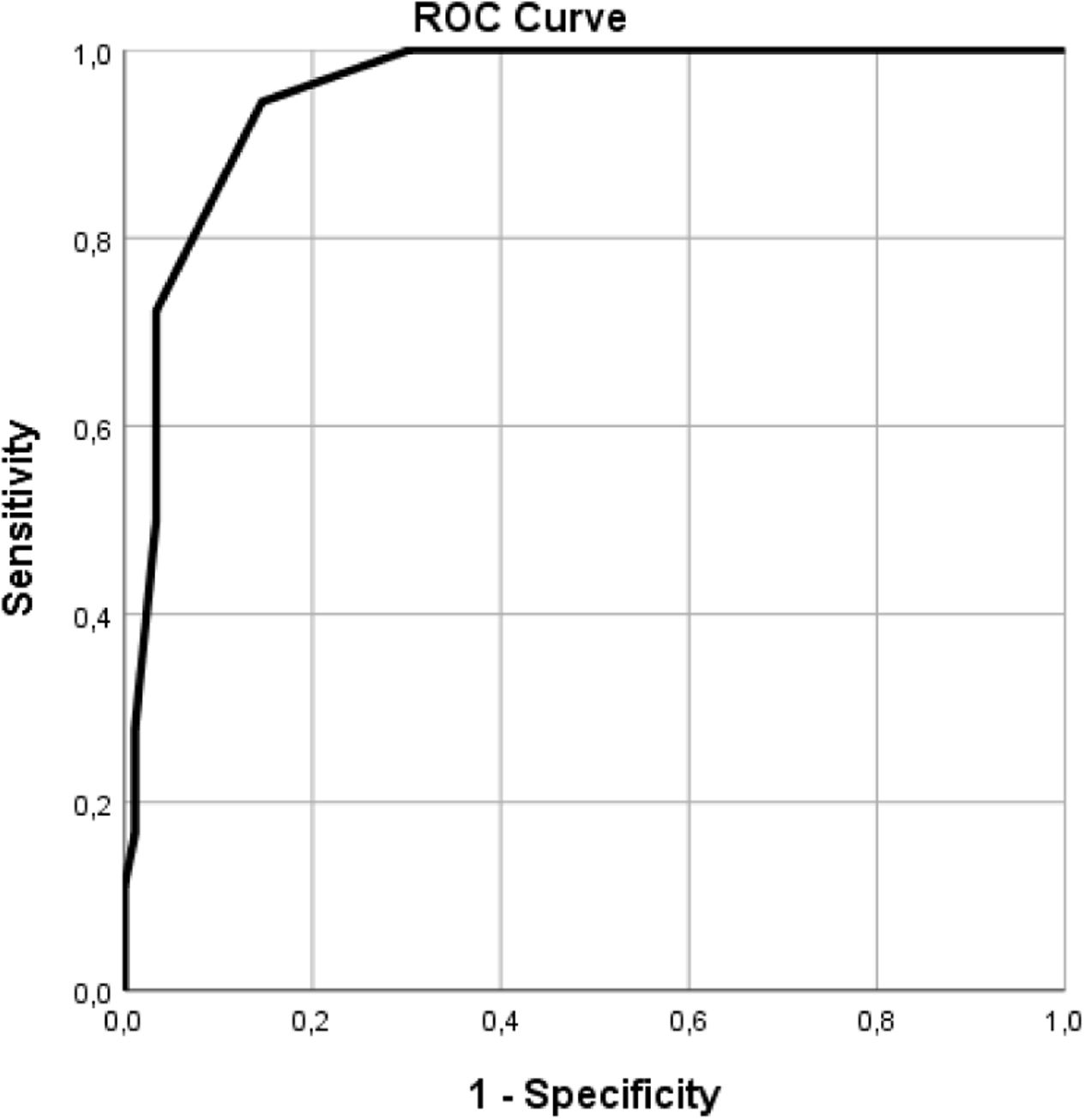
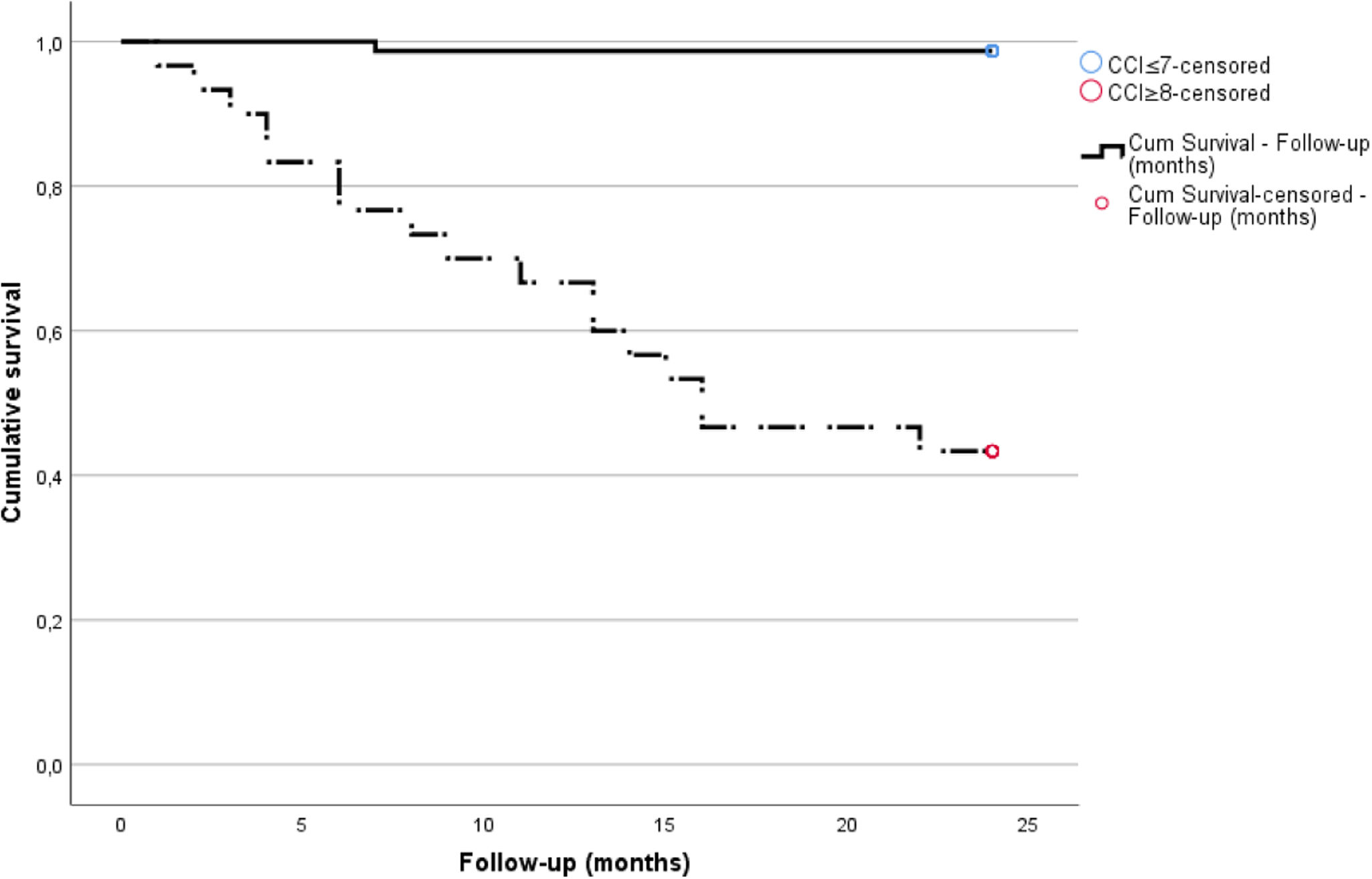
The cohort's one-year and two-year mortality rates were 10.3% (n=11) and 16.8% (n=18), respectively and death occurred on average 9.3±5.7 months post-graft loss. The leading causes of mortality were infection (55.5%, N=10), with four cases of SarsCov-2 pneumonia, followed by CV death (28%, N=5) (Table 2). Table 1 displays the differences in variable distribution for two-year mortality in our cohort. Mortality was significantly associated with older age, lower diuresis, lower albumin levels, higher CFS, higher CCI scores, and hospitalization. The mortality rate was also higher among patients with diabetes, heart failure, high CV risk, and unplanned HD initiation. Transplant-related variables, such as estimated glomerular filtration rate at HD start, months of graft survival, donor characteristics, induction, maintenance IS, and previous graft rejection episodes, did not associate with the two-year mortality rate. Our Cox regression model for mortality achieved statistical significance, explaining 83% of the variance in the studied outcome. It confirmed the relevance of the CCI, CFS, and lower residual diuresis volume in increasing mortality risk (Table 3). After adjusting for confounding variables, a cut-off value for CCI ≥8 (sensitivity 94.4%, specificity 84.9%, area under the curve (AUC) 0.95) was found to predict a higher two-year mortality risk (Fig. 1). Kaplan–Meier curves for patients with a CCI ≥8 (N=30) or ≤7 (N=77) are shown in Fig. 2.
DiscussionTo our knowledge, this study is the first to examine the relationship between frailty, comorbidity, and outcomes in patients with a failing kidney graft who are initiating HD. Over a two-year follow-up period, 37.4% of patients were hospitalized (0.44 events per patient), most commonly due to infection and CV events. Frailty, measured by the CFS, was significantly associated with hospitalization (OR 1.99), highlighting its relevance in predicting post-graft morbidity. In contrast, no significant correlation was found between CCI and hospitalization. Given that the mean time to death was 9.3±5.7 months and that CV-related were the second most common cause, we hypothesize that mortality acted as a competing risk, thereby reducing the likelihood of subsequent hospitalization. Eighteen patients (17%) died during follow-up, most within the first year. Severe infections were the leading cause of death, aligning with previously published data.22 Both CFS and CCI emerged as independent predictors of mortality, as reported in native kidney ESKD.28,29 A cut-off CCI score of ≥8, provided good sensitivity and specificity for two-year mortality risk.
Frailty in ESKD patients with failing grafts is an emerging concern. As graft survival increases and the transplant population ages, comorbidities – particularly CV disease – accumulate.30 In a large study by Gill et al., older age, diabetes, peripheral vascular disease, heart failure, and low serum albumin were the strongest predictors of post-graft failure mortality.22 Other large cohorts have similarly reported high rates of hospitalization and mortality, especially in the first year.8,31,32 However, tools for stratifying patients using frailty and comorbidity scores remain underdeveloped.
Personalizing care for patients with failed allografts has become a key recommendation in recent transplant guidelines.20,21 KT recipients face unique risks due to prolonged IS and complex trajectories of CKD. In a previous work from our group that compared KT recipients with native ESKD patients, we found similar mortality rates despite KT recipients being about 10 years younger – emphasizing the complexity of their risk profile.33
Identifying frail patients at the point of graft failure creates opportunities to intervene early – potentially reducing infection- and CV-related deaths. It also allows tailored discussions around all available post-graft therapies, including conservative kidney management. In native CKD, patients over 80 with high comorbidity and poor functional status may have similar life expectancies, whether managed with dialysis or conservatively.34 We found that older diabetic patients with high CCI scores had a mean survival of only 9 months after starting dialysis, underscoring the importance of considering non-dialysis options. However, none of our patients were referred for conservative care. Managing conservative care post-graft failure presents unique challenges, including ongoing IS.19 While maintaining IS can preserve residual kidney function (RKF) and prevent graft intolerance syndrome – a subclinical inflammatory state that increases morbidity – it also raises risks of infection, malignancy, and metabolic complications.35–37 In our cohort, continued CNI use was not associated with increased hospitalizations or mortality, aligning with recent meta-analyses.38 These findings support individualized IS tapering, with low-dose steroids potentially reducing intolerance syndrome and anuria risk.
Advanced chronic kidney disease units have been established to enhance the quality of life, survival, and morbidity in ESKD.39 The British Transplantation Society recommends dedicated low-clearance transplant clinics offering multidisciplinary tailored care to reduce unplanned dialysis starts and promote early decision-making on dialysis, conservative care, or retransplantation.40,41 More recent studies show better survival rates in patients returning to dialysis, which might reflect the shift of care and the focus on ESKD post-graft failure.42,43 Considering our study results, a low-clearance clinic is being developed for KT patients.
Our study was limited by its retrospective nature and small sample size, including the small number of hospitalization and mortality events that likely affected statistical power. We also lacked data on peritoneal dialysis starts, which may influence outcomes via preserved RKF. Additionally, missing data on induction therapy and donor characteristics further limits the analysis. It is also essential to notice that the study was performed during the SarsCoV-2 pandemic, which had a generally negative impact on the care of chronic patients.44
In conclusion, our findings highlight the importance of frailty and comorbidity in shaping outcomes after kidney graft failure. Patients with a CCI ≥8 face the highest risk of early mortality, particularly in the first-year post-failure. Early identification of at-risk individuals when eGFR declines below 20mL/min/1.73m2 can facilitate smoother transitions, better IS management, tailored symptom control, and proactive retransplantation or conservative care planning.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interestsWe have no conflicts of interest to disclose.