The maturation and patency of permanent vascular access are critical in patients requiring hemodialysis. Although numerus trials have been attempted to achieve permanently patent vascular access, little have been noticeable. Cilostazol, a phosphodiesterase-3 inhibitor, has been shown to be effective in peripheral arterial disease including vascular injury-induced intimal hyperplasia. We therefore aimed to determine the effect of cilostazol on the patency and maturation of permanent vascular access.
MethodsThis single-center, retrospective study included 194 patients who underwent arteriovenous fistula surgery to compare vascular complications between the cilostazol (n=107) and control (n=87) groups.
ResultsThe rate of vascular complications was lower in the cilostazol group than in the control group (36.4% vs. 51.7%; p=0.033), including maturation failure (2.8% vs. 11.5%; p=0.016). The rate of reoperation due to vascular injury after hemodialysis initiation following fistula maturation was also significantly lower in the cilostazol group than in the control group (7.5% vs. 28.7%; p<0.001). However, there were no significant differences in the requirement for percutaneous transluminal angioplasty (PTA), rate of PTA, and the interval from arteriovenous fistula surgery to PTA between the cilostazol and control groups.
ConclusionCilostazol might be beneficial for the maturation of permanent vascular access in patients requiring hemodialysis.
La maduración y la permeabilidad del acceso vascular permanente son fundamentales en los pacientes que requieren hemodiálisis. Aunque se han realizado numerosos ensayos para conseguir un acceso vascular permanentemente permeable, pocos han conseguido resultados destacables. El cilostazol, un inhibidor de la fosfodiesterasa 3, ha demostrado ser eficaz en la enfermedad arterial periférica, incluida la hiperplasia intimal inducida por lesiones vasculares. Por lo tanto, nuestro objetivo era determinar el efecto del cilostazol en la permeabilidad y la maduración del acceso vascular permanente.
MétodosEste estudio unicéntrico y retrospectivo incluyó 194 pacientes sometidos a una cirugía de fístula arteriovenosa para comparar las complicaciones vasculares entre los grupos de cilostazol (n=107) y de control (n=87).
ResultadosLa tasa de complicaciones vasculares fue menor en el grupo de cilostazol que en el grupo de control (36,4% frente a 51,7%; p=0,033), incluido el fracaso de la maduración (2,8% frente a 11,5%; p=0,016). La tasa de reintervención por lesión vascular tras el inicio de la hemodiálisis después de la maduración de la fístula también fue significativamente menor en el grupo de cilostazol que en el grupo de control (7,5% frente a 28,7%; p<0,001). Sin embargo, no hubo diferencias significativas en la necesidad de angioplastia transluminal percutánea (ATP), la tasa de ATP y el intervalo desde la cirugía de la fístula arteriovenosa hasta la ATP entre los grupos de cilostazol y de control.
ConclusiónEl cilostazol podría ser beneficioso para la maduración del acceso vascular permanente en pacientes que necesitan hemodiálisis.
The growth in the number of patients with end-stage renal disease (ESRD) is continuing at a higher rate than the increase in general population, with a consequent increase in the number patients receiving hemodialysis. At the end of 2017, the number of patients receiving renal replacement therapy in Korea was 98746, with 73059 (73.9%) of those receiving hemodialysis.1 This rise in hemodialysis patients is considered to be due to an increase not only in the elderly population but also in those with chronic illnesses such as diabetes and hypertension. Indeed, more than two-thirds of ESRD cases are attributed to chronic diseases such as diabetes and hypertension.
Permanent vascular access is important in patients requiring hemodialysis. Unfortunately, maturation failure, which occurs in 30%–55% of patients undergoing arteriovenous fistula (AVF) surgery for hemodialysis, hinders the utility of AVF.2–4 Delayed complications of mature fistulas result in reinterventions and hospital admissions as well as a one-year fistula failure rate of 30%–40%.4–8
Intimal hyperplasia (IH) is a vasculopathy characterized by the differentiation of smooth muscle cells that form a multilayer compartment in the tunica intima of blood vessels. Direct endothelial damage is the most important cause of IH in coronary artery disease and peripheral arterial disease.9–11 Conversely, both direct vascular damage due to hypertension and indirect vascular injury due to uremia, anemia, or chronic inflammation are responsible for IH in chronic kidney disease.12 The effects of cilostazol on direct vascular injury-induced IH have been previously demonstrated,9–11 whereas the impact of cilostazol on indirect vascular injury-induced IH in patients with chronic kidney disease remains unclear.12 In the present retrospective study, we tested the hypothesis that cilostazol might improve the maturation rate and durability of vascular access for hemodialysis.
Materials and methodsStudy populationThis single-center, retrospective study was approved by the institutional review board of Chungnam National University Hospital (IRB No. 2019-05-039) and included patients undergoing AVF surgery between January 2012 and June 2018 at Chungnam National University Hospital in Daejeon, South Korea.
Patients fulfilling the following criteria were included in the study: (1) more than two months of treatment with cilostazol, (2) hemodialysis initiation at Chungnam National University Hospital, (3) AVF surgery performed at Chungnam National University Hospital, (4) age of more than 18 years, (5) post-AVF surgery follow-up of six months or more, and (6) hemodialysis for more than six months. The exclusion criteria were (1) discontinuation of hemodialysis due to improvement in acute kidney injury within six months after the initiation of hemodialysis, (2) no initiation of hemodialysis within six months after AVF surgery, (3) less than two months of treatment with cilostazol, and (4) previous AVF surgery.
Forty-three patients received hemodialysis prior to 2012 and underwent reoperation at the study hospital between January 2012 and June 2018, whereas 95 patients were followed in our clinic for less than six months after surgery. Ten patients treated with cilostazol for less than two months were excluded from the study. Of a total of 348 patients, 194 and 154 patients underwent AVF and arteriovenous graft (AVG) surgery, respectively, and the present study included 194 patients who underwent AVF surgery.
Measurements and definitionsThe medical records of patients were reviewed to collect data on age, sex, underlying diseases, cause of ESRD (e.g., diabetes mellitus, hypertension, glomerulonephritis, other), follow-up duration, date of hemodialysis initiation, date of AVF surgery, whether treatment with cilostazol, duration of cilostazol treatment, treatment with other anticoagulants (aspirin, clopidogrel, warfarin, sarpogrelate), presence of peripheral arterial disease, vascular complications (e.g., maturation failure, radiological and surgical interventions to maintain patency), number of percutaneous transluminal angioplasty (PTA) procedures, and interval from AVF surgery to PTA.
The cilostazol group included patients who started cilostazol treatment before or within three days after surgery and those who were treated with cilostazol for more than two months, whereas the control group included patients who were not treated with cilostazol within the period between six months before and after surgery. The study cohort of 194 patients who underwent AVF surgery included 107 and 87 patients in the cilostazol and control groups, respectively.
Vascular complications were defined as serious vascular access events and included maturation failure, PTA, and reoperation after fistula maturation. Maturation failure was defined as requirement for reoperation due to an AVF that could not be used clinically after surgery or one that required PTA. PTA was defined as the presence of stenosis or thrombosis of an AVF after surgery requiring intervention. Reoperation after fistula maturation was defined as the clinical necessity for a second AVF or AVG surgery in patients undergoing hemodialysis after the first AVF surgery.
Primary outcome was vascular complications, and secondary outcomes were number of PTA procedures and interval between PTA and vascular surgery (i.e., complication-free days).
Statistical analysisContinuous variables were analyzed using Student's t test, and categorical variables were analyzed using Pearson's chi-square test. Continuous variables are reported as means with standard deviation, whereas discrete variables are reported as percentages (%). Differences in the duration of follow-up between the groups were evaluated using the Student's t-test. Survival analysis for vascular complication was performed by Kaplan–Meier curve analysis. All analyses were performed using the SPSS statistics software version 20 (IBM, Armonk, NY, USA), and p values of less than 0.05 were considered to indicate statistical significance.
ResultsThe patient demographics were similar between the cilostazol and control groups (Table 1). Specifically, the mean ages of the cilostazol and control groups were 64.3 and 65.0 years, respectively, which was not significantly different (p=0.726). Furthermore, there were no differences in comorbidities, other medications including antiplatelet agents and anticoagulation agents, and cause of ESRD between the two groups.
Demographic and clinical characteristics of patients undergoing AVF surgery.
| Cilostazol group (n=107) | Control group (n=87) | p value | |
|---|---|---|---|
| Demographic characteristics | |||
| Male | 79 (73.8%) | 60 (69.0%) | 0.455 |
| Age (years) | 64.3±12.3 | 65.0±12.6 | 0.726 |
| Death | 12 (11.2%) | 13 (14.9%) | 0.441 |
| Follow-up duration (days) | 704.1±458.0 | 940.9±575.1 | 0.002 |
| Antiplatelet/anticoagulant agents | |||
| Aspirin | 26 (24.3%) | 21 (24.1%) | 0.979 |
| Clopidogrel | 21 (19.6%) | 27 (31.0%) | 0.067 |
| Warfarin | 2 (1.9%) | 1 (1.1%) | 0.686 |
| Sarpogrelate | 9 (8.4%) | 6 (6.9%) | 0.694 |
| Comorbidity | |||
| Diabetes | 77 (72.0%) | 64 (73.6%) | 0.803 |
| Hypertension | 92 (86.0%) | 68 (78.2%) | 0.154 |
| GN | 9 (8.4%) | 10 (11.5%) | 0.472 |
| PAD | 10 (9.3%) | 12 (13.8%) | 0.331 |
| PAD with PTA | 6 (5.6%) | 10 (11.5%) | 0.138 |
| CAD | 33 (30.8%) | 31 (28.7%) | 0.480 |
| HF | 29 (27.1%) | 24 (27.6%) | 0.940 |
| A.fib/A.flutter | 9 (8.4%) | 8 (7.6%) | 0.848 |
| CVA | 30 (28.0%) | 34 (39.1%) | 0.104 |
| Cause of ESRD | 0.686 | ||
| Diabetes | 74 (69.2%) | 57 (65.5%) | |
| Hypertension | 22 (20.6%) | 16 (18.4%) | |
| GN | 6 (5.6%) | 8 (9.2%) | |
| Others | 5 (4.7%) | 6 (6.9%) | |
Afib, atrial fibrillation; A. flutter, atrial flutter; CAD, coronary artery disease; CVA, cerebrovascular accident; GN, glomerulonephritis; HF, heart failure (ejection fraction<50%); PAD, peripheral artery disease; PTA, percutaneous transluminal angioplasty.
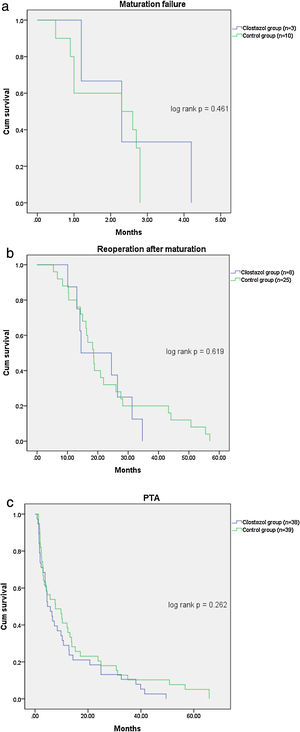
Table 2 shows the patient outcomes between the cilostazol and control groups. The vascular complications were significantly lower in the cilostazol group than in the control group (36.4% [n=39] vs. 51.7% [n=45]; odds ratio [OR] 0.535, 95% confidence interval [CI] 0.301–0.952, p=0.033). The analysis of the vascular complications by subgroups revealed that the rate of maturation failure was significantly lower in the cilostazol group than in the control group (2.8% [n=3] vs. 11.5% [n=10], OR 0.222, 95% CI 0.059–0.834, p=0.016) and that the number of patients undergoing hemodialysis for reoperation after fistula maturation was significantly lower in the cilostazol group than in the control group (7.5% [n=8] vs. 28.7% [n=25], OR 0.200, 95% CI 0.085–0.472, p<0.001). The rate of radiological interventions (PTA) to maintain fistula patency was also lower in the cilostazol group than in the control group, although the difference was not statistically significant (35.5% [n=38] vs. 44.8% [n=39], OR 0.678, 95% CI 0.380–1.209, p=0.187). Furthermore, the number of PTA procedures was not significantly different between the cilostazol (1.7±1.1) and control (2.2±2.0) groups (p=0.157). Finally, there were no statistically significant differences the interval between AVF surgery and PTA between the two groups (p=0.300). The incidence of vascular complication was a significant difference in maturation failure and reoperation after maturation, but there was no statistically significant difference in survival analysis (Fig. 1).
Comparison of vascular complications following AVF surgery between the cilostazol and control groups.
| Cilostazol group (n=107) | Control group (n=87) | p value | |
|---|---|---|---|
| Vascular complications | 39 (36.4%) | 45 (51.7%) | 0.033 |
| Maturation failure | 3 (2.8%) | 10 (11.5%) | 0.016 |
| Reoperation after maturation | 8 (7.5%) | 25 (28.7%) | <0.001 |
| PTA (radiological interventions) after maturation | 38 (35.5%) | 39 (44.8%) | 0.187 |
| Number of PTA procedures | 1.7±1.1 | 2.2±2.0 | 0.157 |
| Interval between first PTA and vascular surgery (days) | 337.4±394.7 | 450.9±543.2 | 0.300 |
PTA, percutaneous transluminal angioplasty.
Of the 194 AVF surgical patients, 100 patients underwent radiocephalic (RC) fistula surgery, 93 patients underwent brachiocephalic (BC) fistula surgery, and 1 patient underwent brachiobasilic fistula surgery. When comparing the cilostazol group and the control group in patients that underwent RC fistula surgery, vascular complications were significantly lower in the cilostazol group than in the control group (39.3% [n=22] vs. 61.4% [n=27], p=0.028), and reoperation after fistula maturation was also significantly lower in the cilostazol group than in the control group (5.4% [n=3] vs. 27.3% [n=12], p=0.002). The rates of maturation failure (p=0.249) and PTA (p=0.081) were not significantly different between the cilostazol group and the control group. When comparing the cilostazol group and control group in patients that underwent BC fistula surgery, there was no significant difference in the rate of vascular complications between the two groups (33.3% [n=17] vs. 42.9% [n=19], p=0.345). However, the rates of maturation failure (2.0% [n=1] vs. 14.3% [n=6], p=0.043) and reoperation after fistula maturation (9.8% [n=5] vs. 31.0% [n=13], p=0.010) were significantly lower in the cilostazol group than in the control group. The rate of PTA to maintain fistula patency was not significantly different between the two groups (p=0.840) (Table 3).
Comparison of vascular complications following AVF site between the cilostazol and control groups between the cilostazol and control groups.
| Radiocephalic fistula (n=100) | Brachiocephalic fistula (n=93) | |||||
|---|---|---|---|---|---|---|
| Cilostazol group (n=56) | Control group (n=44) | p value | Cilostazol group (n=51) | Control group (n=42) | p value | |
| Vascular complications | 22 (39.3%) | 27 (61.4%) | 0.028 | 17 (33.3%) | 18 (42.9%) | 0.345 |
| Maturation failure | 2 (3.6%) | 4 (9.1%) | 0.401a | 1 (2.0%) | 6 (14.3%) | 0.043a |
| Reoperation after maturation | 3 (5.4%) | 12 (27.3%) | 0.002 | 5 (9.8%) | 13 (31.0%) | 0.010 |
| PTA (radiological interventions) after maturation | 22 (39.3%) | 25 (56.8%) | 0.081 | 16 (31.4%) | 14 (33.3%) | 0.840 |
| Number of PTA procedures | 1.6±0.9 | 2.1±1.4 | 0.107 | 1.9±1.4 | 2.4±2.7 | 0.533 |
| Interval between first PTA and vascular surgery (days) | 307.0±421.4 | 512.1±596.3 | 0.181 | 358.9±362.1 | 230.9±317.1 | 0.290 |
PTA, percutaneous transluminal angioplasty.
The present single-center, retrospective study was designed to evaluate the potential benefit of cilostazol in patients with hemodialysis access. Several common mechanisms underlie the pathophysiology of AVF maturation failure and vascular complications for all vascular access types, particularly those involved in neointimal hyperplasia.17–20 Studies have consistently demonstrated that changes in the extracellular matrix, which occur early during vascular remodeling, can lead to failed fistula dilation and subsequent stenosis and thrombosis of the access point. A large randomized controlled trial has demonstrated that thrombosis plays only a limited role in failed fistula maturation and vascular remodeling and that IH is a more prominent mechanism.17
The pathophysiology of IH is complex, but one central hemodynamic-related mechanism involves the upregulation of mitogen-activated protein kinases.18 Experimental studies suggest that this pathway may be controlled by cilostazol,19–21 a phosphodiesterase (PDE)-3 inhibitor that is used for the treatment of intermittent claudication.13,15,16,22-24 PDE-3A and PDE-3B are two members of the PDE-3 enzyme family. PDE-3A is expressed in platelets, vascular smooth muscle cells, and cardiomyocytes, whereas PDE-3B is found in adipocytes, hepatocytes, pancreatic β-cells, and macrophages.14,25 Therefore, the pharmacological effects of cilostazol include vasodilation, inhibition of platelet activation and aggregation, inhibition of thrombosis, increased blood flow to extremities, improvement in serum lipids, and inhibition of vascular smooth muscle cell growth; these effects of cilostazol contribute to the reduction of AVF maturation failure.17 The use of cilostazol after vascular surgery may reduce the incidence of maturation failure and subsequent intervention or reoperation by influencing vascular remodeling, in agreement with the results of the present study.17
The main finding of the present study was the reduction in vascular complications among hemodialysis patients treated with cilostazol. Specifically, the incidence of maturation failure was significantly lower in the cilostazol-treated patients who underwent hemodialysis without maturation failure after vascular surgery. Additionally, the incidence of reoperation was also significantly lower in the patients treated with cilostazol. However, there was no significant difference in vascular complication in survival analysis using Kaplan-Meier curve analysis. It is thought that maturation failure was mostly determined within 2 months after surgery, so it seems that there was no difference in the time factor.
When comparing the vascular complications according to the fistula location, patients that underwent RC fistula surgery experienced fewer vascular complications when taking cilostazol. The analysis of the vascular complications by subgroups revealed that the rate of reoperation after fistula maturation was significantly lower in the cilostazol group than in the control group. However, there were no significant differences in the rates of maturation failure and PTA between the cilostazol and control groups. Patients that underwent BC fistula surgery did not experience fewer vascular complications when taking cilostazol. However, the analysis of the vascular complications by subgroups revealed that the rates of maturation failure and reoperation were significantly lower in the cilostazol group than in the control group. The rate of PTA was not significantly different between the two groups in patients that underwent BC fistula surgery. When cilostazol was administered, the rate of reoperation after fistula maturation was significantly lower in both RC fistula surgery and BC fistula surgery, the rate of maturation failure was significantly lower in BC fistula surgery, not RC fistula surgery. In our study, it is difficult to analyze the preoperative vein diameter because there was no measurement and vascular ultrasound reports, but in the previous study, the vein diameter was found to be an independent predictor of maturation.26 Therefore, in patients that underwent RC fistula surgery, the vein diameter was generally smaller than in patients that underwent BC fistula surgery, so it is expected that these factors may have influenced maturation failure. Therefore, when the vein diameter is large, the effect of cilostazol can be more clearly evaluated, and when the vein diameter is small, it might be difficult to compare the effects of cilostazol because the vein diameter can be a risk factor for maturation failure. In patients that underwent BC fistula surgery, the rates of both maturation failure and reoperation were lower in the cilostazol group, although the rate of vascular complications was not different between the cilostazol group and the control group. In our study, vascular complications were defined as serious vascular access events, including maturation failure, PTA, and reoperation after fistula maturation. In patients who underwent BC fistula surgery, there was no difference in the rate of PTA between the cilostazol group and the control group, and this effect showed no difference in vascular complications.
As cilostazol is a PDE-3 inhibitor, it is expected to reduce the incidence of maturation failure and reoperation by inhibiting IH by reducing vascular smooth muscle cell growth after AVF surgery.9–11 However, if PTA is performed owing to stenosis or thrombosis of AVF after maturation, it may occur due to mechanical trauma from venipuncture or shear stress due to turbulent blood flow rather than IH.27,28 Therefore, administration of cilostazol is considered to not have a significant influence on PTA.
The present study has several limitations that should be acknowledged. First, the patient population was relatively small in this single-institution study. However, the study cohort was larger than that of a previously published similar study.17 Additionally, between January 2012 and June 2018, only one surgeon performed AVF and AVG surgeries which might reduce bias due to differences in surgical skills among different surgeons. The present study did not reveal a difference in the number of PTA procedures between the cilostazol and control groups. Since vascular conditions that require PTA can cause endothelial damage and IH, cilostazol administration might affect the final number of patients undergoing PTA. However, the cilostazol and control groups did not exhibit differences in the efficacy of cilostazol after PTA in the present study and a future controlled study is warranted.
In conclusion, the present retrospective, single-surgeon, single-center study found that there were differences in vascular complications between the patients treated with cilostazol and those who were not administered cilostazol; however, a definitive conclusion requires a larger study cohort. Future well-designed prospective studies with larger populations are necessary to address these issues.
Conflict of interestAuthors declare no conflict of interest.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1AB03035061).