After reading some recent reports on the appearance or de novo recurrence of different glomerulopathies after the application of vaccines against COVID-191–10 we would like to contribute our experience.
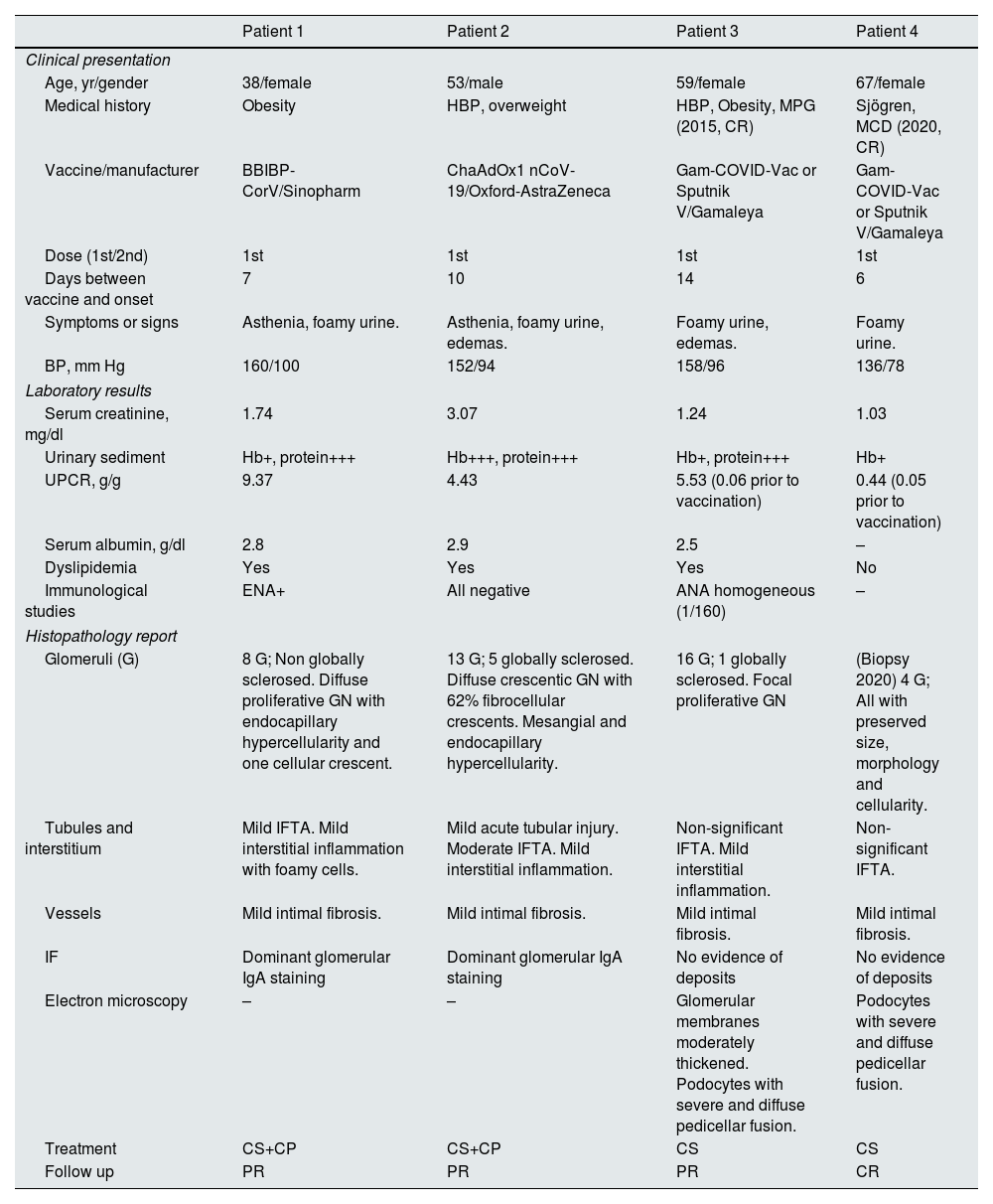
Four patients (P) previously asymptomatic began with asthenia (P1, P2), foamy urine (all) and edema (P2, P3) within 2 weeks after the first dose of the COVID-19 vaccine (P1: Sinopharm, P2: Oxford-AstraZeneca, P3 and P4: Gamaleya). All presented hypertension and microscopic hematuria. The first three patients also presented with acute kidney injury, nephrotic range proteinuria, hypoalbuminemia and dyslipidemia. The P4 increased his proteinuria significantly. Renal biopsies performed showed two IgA nephropathy with crescent (Supplementary Fig. S1, A–F) and recurrence of proliferative glomerulonephritis (Supplementary Fig. S2, A and B). P4 did not undergo a new biopsy since she had a previous diagnosis in 2020 of minimal change disease (Supplementary Fig. S2, C and D) and it was interpreted as the beginning of a recurrence of her disease. All received corticosteroids and P1 and P2 also received cyclophosphamide. P1, P2 and P3 presented partial remission and P4 complete remission (Table 1).
Patient demographics and clinical characteristics.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Clinical presentation | ||||
| Age, yr/gender | 38/female | 53/male | 59/female | 67/female |
| Medical history | Obesity | HBP, overweight | HBP, Obesity, MPG (2015, CR) | Sjögren, MCD (2020, CR) |
| Vaccine/manufacturer | BBIBP-CorV/Sinopharm | ChaAdOx1 nCoV-19/Oxford-AstraZeneca | Gam-COVID-Vac or Sputnik V/Gamaleya | Gam-COVID-Vac or Sputnik V/Gamaleya |
| Dose (1st/2nd) | 1st | 1st | 1st | 1st |
| Days between vaccine and onset | 7 | 10 | 14 | 6 |
| Symptoms or signs | Asthenia, foamy urine. | Asthenia, foamy urine, edemas. | Foamy urine, edemas. | Foamy urine. |
| BP, mm Hg | 160/100 | 152/94 | 158/96 | 136/78 |
| Laboratory results | ||||
| Serum creatinine, mg/dl | 1.74 | 3.07 | 1.24 | 1.03 |
| Urinary sediment | Hb+, protein+++ | Hb+++, protein+++ | Hb+, protein+++ | Hb+ |
| UPCR, g/g | 9.37 | 4.43 | 5.53 (0.06 prior to vaccination) | 0.44 (0.05 prior to vaccination) |
| Serum albumin, g/dl | 2.8 | 2.9 | 2.5 | – |
| Dyslipidemia | Yes | Yes | Yes | No |
| Immunological studies | ENA+ | All negative | ANA homogeneous (1/160) | – |
| Histopathology report | ||||
| Glomeruli (G) | 8 G; Non globally sclerosed. Diffuse proliferative GN with endocapillary hypercellularity and one cellular crescent. | 13 G; 5 globally sclerosed. Diffuse crescentic GN with 62% fibrocellular crescents. Mesangial and endocapillary hypercellularity. | 16 G; 1 globally sclerosed. Focal proliferative GN | (Biopsy 2020) 4 G; All with preserved size, morphology and cellularity. |
| Tubules and interstitium | Mild IFTA. Mild interstitial inflammation with foamy cells. | Mild acute tubular injury. Moderate IFTA. Mild interstitial inflammation. | Non-significant IFTA. Mild interstitial inflammation. | Non-significant IFTA. |
| Vessels | Mild intimal fibrosis. | Mild intimal fibrosis. | Mild intimal fibrosis. | Mild intimal fibrosis. |
| IF | Dominant glomerular IgA staining | Dominant glomerular IgA staining | No evidence of deposits | No evidence of deposits |
| Electron microscopy | – | – | Glomerular membranes moderately thickened. Podocytes with severe and diffuse pedicellar fusion. | Podocytes with severe and diffuse pedicellar fusion. |
| Treatment | CS+CP | CS+CP | CS | CS |
| Follow up | PR | PR | PR | CR |
ANA, antinuclear antibodies; CP, cyclophosphamide; CR, complete remission; CS, corticosteroids; ENA, antibodies against extractable nuclear antigens; GN, glomerulonephritis; HBP, high blood pressure; Hb, hemoglobin; IFTA, interstitial fibrosis and tubular atrophy; MPG, mesangial proliferative glomerulonephritis; MCD, minimal change disease; PR, parcial remission; UPCR, urine protein-to-creatinine ratio.
Although it is very difficult to prove causation, at the time of this letter there are at least 40 reports of different types of glomerulopathies after receiving the COVID-19 vaccine from different manufacturers (Pfizer, Moderna, AstraZeneca and Sinovac).1–10 To our knowledge, our reports would be the first related to the Sputnik V (Gamaleya) and BBIBP-CorV (Sinopharm) vaccines. Undoubtedly, the benefits of vaccines far outweigh the risks, but these findings emphasize the importance of surveillance in patients with previous glomerulonephritis and/or the appearance of foamy urine, edemas, hypertension or laboratory abnormalities.
FundingThis work has not received any type of funding.
Conflict of interestThe author declares that he has no conflict of interest.