Iron deficiency (ID) is highly prevalent in chronic kidney disease (CKD) and it's associated with poorer quality of life (QoL) and functional capacity. Intravenous iron therapy is limited to CKD patients with ID and anemia to avoid/delay the need or reduce the dose of erythropoiesis-stimulating agents, according to guidelines. We hypothesized that treatment with IV iron in CKD patients with ID and borderline anemia may improve their physical performance and QoL, independently of its effects on hemoglobin.
MethodsProspective, single-arm study in CKD patients with ID and mild anemia. The 6-min walk test (6-MWT), Piper fatigue scale, Patient's global assessment (PGA) and QoL (EQ-5D) questionnaires were evaluated at baseline, and at weeks 1 and 4 after receiving IV ferric carboxymaltose. Changes in continuous endpoints and their longitudinal trajectories were estimated with linear mixed regression models (LMRMs).
ResultsForty-one patients completed the study. The 6-MWT increased from 296±101m to 314±106m at week 1 (p<0.01), and to 325±111 meters at week 4 (p<0.01). PGA, EQ-5D questionnaire and Piper Fatigue scale significantly improved at week 4 from baseline (p<0.05), after adjustment in the last 2 variables. Hemoglobin levels did not increase significantly during the follow-up.
ConclusionsIV ferric carboxymaltose (IV FCM) was associated with a significant short-term improvement in the 6-MWT in CKD patients with iron deficiency and mild anemia. PGA, EQ-5D and Piper Fatigue Scale also improved at 4 weeks. These findings suggest a potential short-term benefit of IV ferric carboxymaltose on physical performance and PROMs in this population, independent of hemoglobin changes; however, given the small sample size and absence of a control group, results should be interpreted with caution and considered hypothesis-generating.
El déficit de hierro (DH) es altamente prevalente en la enfermedad renal crónica (ERC), y se asocia con una peor calidad de vida y capacidad funcional. Según las guías clínicas, el tratamiento con hierro intravenoso está limitado a los pacientes con ERC que presentan DH y anemia, con el objetivo de evitar o retrasar la necesidad de agentes estimulantes de la eritropoyesis, o reducir su dosis. Nuestra hipótesis fue que el tratamiento con hierro intravenoso en los pacientes con ERC, DH y anemia leve podría mejorar su rendimiento físico y calidad de vida, independientemente de su efecto sobre la hemoglobina.
MétodosEstudio prospectivo, de un solo brazo, en los pacientes con ERC, ID y anemia leve. Se evaluaron el test de marcha de 6min (6-MWT), la escala de fatiga de Piper, la evaluación global del paciente (PGA) y los cuestionarios de calidad de vida (EQ-5D) al inicio y en las semanas 1 y 4 tras la administración de carboximaltosa férrica intravenosa. Los cambios en los desenlaces continuos y sus trayectorias longitudinales se estimaron mediante modelos de regresión lineal mixta (LMRM).
ResultadosCuarenta y un pacientes completaron el estudio. La distancia recorrida en el 6-MWT aumentó de 296±101 a 314±106m en la semana 1 (p<0,01), y a 325±111m en la semana 4 (p<0,01). La PGA, el cuestionario EQ-5D y la escala de fatiga de Piper mejoraron significativamente en la semana 4 respecto al inicio (p<0,05), tras ajustes en las dos últimas variables. Los niveles de hemoglobina no aumentaron significativamente durante el seguimiento.
ConclusionesLa administración intravenosa de carboximaltosa férrica se asoció con una mejora significativa a corto plazo en el test de marcha de 6 minutos en pacientes con ERC e DH La PGA, el EQ-5D y la escala de fatiga de Piper también mostraron mejoría a las 4 semanas. Estos hallazgos respaldan el papel de la deficiencia de hierro en el deterioro del rendimiento físico en pacientes con ERC.
Anemia is a frequent complication in chronic kidney disease (CKD) patients1,2 while iron deficiency (ID) is a common cause of anemia in CKD and is highly prevalent in this population, independent of the CKD stage.3 Current clinical guidelines on anemia in CKD recommend iron therapy only when ID and anemia coexist4–6 but there are no indications for correction of ID in CKD, independently of the presence of anemia.
Iron is involved in vital cellular and body functions, including oxygen transport and storage (hemoglobin and myoglobin), energy production, DNA synthesis and repair, immune function, among others.7 In fact, clinical symptoms in patients with CKD, often attributed to anemia, may also be due to ID. Prior studies have shown that ID in CKD is associated with an increased risk of morbidity and mortality and a worse quality of life (QoL), regardless of the presence of anemia.8–10 In patients with heart failure (HF) with reduced ejection fraction and ID, intravenous (IV) iron supplementation improved functional status, QoL, and reduced the risk of HF hospitalization in patients with and without anemia.11–14
Two randomized controlled trials of IV iron vs placebo in ID non-anemic non-dialysis CKD (ND-CKD) patients have recently been published.15,16 Both trials failed to demonstrate the superiority of ID correction with IV iron in improving the functional capacity or the QoL. However, patients were relatively younger than current non-dialysis CKD patients and had a relatively good baseline physical status.
We hypothesized that treating ID with IV iron in an unselected mildly anemic ND-CKD population would result in short-term improvements in functional capacity and other patient-reported outcomes (PROMs), independent of hemoglobin levels. The 6-minute walk test (6-MWT) is a reliable tool for assessing functional capacity, as it predicts peak oxygen uptake in hemodialysis patients.17 To test this, we evaluated the early effects of IV ferric carboxymaltose (FCM) on functional capacity and other PROMs in ND-CKD patients with mild anemia and ID
Patients and methodsStudy designWe conducted a 4-week, single-center, open-label, prospective, single-arm, pilot study to assess the effect of IV ferric carboxymaltose (FCM) on functional capacity and QoL in ND-CKD patients with mild anemia and ID.
PatientsFrom May 2021 to June 2022, 45 eligible patients were enrolled in the study. The inclusion criteria were: adults, ND-CKD patients stages 3–5, ID (ferritin<100ng/ml or ferritin<200ng/ml if TSAT<20%) and mild anemia (Hb 10.5–11.5g/dL).
The exclusion criteria were: anemia due to reasons other than ID and CKD (e.g. hemoglobinopathy), having received erythropoiesis-estimulating agents (ESA) therapy, IV iron and/or blood transfusion in the previous 2 months; clinical heart failure, or C-reactive protein>20mg/L, among others (see Supplementary material).
Study proceduresPatients were recruited from the Nephrology outpatient's clinic. Forty five patients were included in the study, four of them did not complete the protocol and 41 patients were finally analyzed.
After screening, patients were required to attend visits at weeks 0 (baseline visit), 1 (day 7th) and 4 (day 28th). At each visit a blood sample was collected for biochemical and hematological measurements. In addition, the 6MWT, Patient's global Assessment (PGA), QoL (EQ-5D questionnaire), and Piper fatigue scale18 were completed at all visits. The questionnaires were self-reported by the patients. At the baseline visit, after all the studies were performed, IV FCM was administered in a single dose, according to the degree of ID calculated by the Ganzoni's formula (Total iron deficiency [mg]=body weight [kg]×(Hb objective−current Hb) [g/L]×0.24*+iron stores [mg].19 The calculation was made considering a target Hb of 12g/dL.
The 6MWT was performed in an area of the hospital equipped for cardiopulmonary resuscitation, along a flat, straight corridor with a hard surface under the supervision of a trained nurse study coordinator, as previously described.
Laboratory results, including hematological parameters, iron status (serum iron, ferritin, transferrin saturation index [TSAT]) and blood biochemistry, including serum urea, creatinine, estimated glomerular filtration rate (eGFR, CKD-EPI formula) and phosphate levels, were evaluated at baseline and follow-up visits (week 1 and week 4) (Fig. 1). During the study, patients were instructed not to take any potential additional treatment for anemia, such as vitamins or nutritional supplements.
Ethical and regulatory issuesThe study was conducted according to the principles of the Declaration of Helsinki and the ICH guidelines for Good Clinical Practices. The protocol was approved by the Ethics Committee for Research with Medicines of the Hospital Clínico Universitario de Valencia. All patients signed the informed written consent prior to any study-related procedures.
Study endpointsThe co-primary endpoint for the study was the change in the 6MWT distance covered from baseline to week 1 and at week 4 after receiving IV FCM.
Secondary endpoints were: changes in PGA, EQ-5D QoL questionnaire and Piper fatigue scale at week 4 from baseline.
Changes in hemoglobin, iron status, eGFR, and serum phosphorus levels at weeks 1 and 4 from baseline were considered as exploratory variables. Safety data were also recorded.
Sample size calculationA repeated-measures ANOVA was considered to calculate the number of patients needed to find statistically significant differences. In the absence of single-arm studies, the sample size calculation was based on previous placebo-controlled studies on a heart failure population where the primary endpoint assessed by the 6MWT was based on functional capacity at 1 and 3 months after receiving a single dose of iv iron. This required 40 participants to detect a 20 meters increase in the 6MWT between 4 weeks and the baseline, with 80% power and a type I error rate of 5% for a high effect size.11
Statistical analysisAs appropriate, continuous baseline variables were expressed as mean±standard deviation or median and interquartile range (IQR). Discrete variables were presented as numbers (percentages). When the p-value of either test was below the 5% significance level, post hoc tests were conducted for determining which pairs of groups (time points) were significantly different and the Benjamini–Hochberg (BH) method20 was used for controlling the false discovery rate. The Student's t-test for parametric variables and the Wilcoxon test for nonparametric variables were used as post hoc tests.
Changes in continuous endpoints and their longitudinal trajectories were estimated with LMRMs. Multivariate estimates were adjusted for age, sex, eGFR, hemoglobin, and the baseline endpoint value regardless of their p-value. The LMRMs are presented as least square means (LSM) with their respective 95% confidence intervals. p-Values were adjusted for multiple comparisons (Sidak procedure). A 2-sided p-value<0.05 was considered statistically significant.
All statistical analyses were done with the R software (version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria) andStata 15.1 (Stata Statistical Software, Release 15 [2017]; StataCorp LP, College Station, TX, USA).
ResultsOf the 45 patients who received IV FCM, 4 did not complete the follow-up (one due to a COVID-19 infection, one due to a chronic obstructive pulmonary disease exacerbation, one due to a hip fracture, and one due to a change of residence). The demographic and clinical baseline characteristics of the 41 patients included are shown in Table 1. The mean dose of IV FCM administered was 616.2±163.8mg.
Baseline characteristics of the patients.
| Variables | Study population(n=41) |
|---|---|
| Age (years), mean±SD | 74.9±9.5 |
| Gender, (%) | |
| Male | 27 (66%) |
| Female | 14 (34%) |
| CKD stage (%) | |
| 3 | 24 (59%) |
| 4 | 17 (41%) |
| Comorbidities | |
| Hypertension, n (%) | 39 (95) |
| Type 2 diabetes mellitus, n (%) | 32 (80) |
| Cardiovascular disease, n (%) | 10 (24) |
| Coronary heart disease, n (%) | 3 (7.3) |
| Peripheral vascular disease, n (%) | 5 (12.2) |
| Cerebrovascular disease (%) | 0 (0) |
| Weight (kg) | 74.4±10.6 |
| Body mass index (kg/m2) | 29.1±4.8 |
| Blood pressure (mm Hg) | |
| Systolic | 142±16.6 |
| Diastolic | 74.0±8.8 |
| Heart rate, beats per minute | 73±11 |
| Treatments | |
| ACEI, n (%) | 5 (12) |
| ARBs, n (%) | 27 (65.9) |
| Calcium channel blockers, n (%) | 21 (51.2) |
| Beta blockers, n (%) | 21 (51.2) |
| Diuretics, n (%) | 31 (75.6) |
| MRA, n (%) | 4 (9.8) |
| Alfa-blockers, n (%) | 14 (34) |
| Potassium binders, n (%) | 5 (12.2) |
| Vitamin D, n (%) | 8 (19.5) |
| Statins, n (%) | 36 (87.8) |
| Antiplatelet agents, n (%) | 18 (43.9) |
| Oral anticoagulants, n (%) | 9 (9.5) |
| SGLT2i, n (%) | 8 (9.5) |
| Metformin, n (%) | 13 (31.7) |
| GLP 1-RA, n (%) | 5 (12.2) |
| Insulin, n (%) | 11 (26.8) |
| DPP4-i, n (%) | 19 (46.3) |
ACEI, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; MRA, mineralcorticoid receptor antagonists; SGLT2i, sodium-glucose cotransporter type 2 inhibitors; GLP1-RA, glucagon-like peptide-1 receptor agonists; DPP4-i, dipeptidyl peptidase 4 inhibitors.
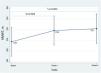
After the infusion of IV FCM, there was an improvement in the distance covered in the 6MWT both at week 1 and at week 4. (Raw data is presented in Table 1 of supplementary material). Inferential analysis confirmed the improvement of the distance walked in 6min after IV FCM administration at the two time points as shown in Fig. 2. LSM [95% confidence interval] for baseline distance completed was 297.27 [269.83–324.71]m. At week 1 and week 4 there was an increase in the meters walked reaching 318.3 [290.71–345.98] and 321.7 [293.92–349.56] respectively (p=0.006 week 1 vs baseline and p=0.002 week 4 vs baseline).
Secondary end-points: changes in PGA, EQ-5D and Piper fatigue scaleFor the secondary end points raw data are presented in Table 1 supplementary material. In the inferential analysis we did not find significant improvements in these tests at week 1, but there were significant improvements of the scores at week 4 of all questionnaires (PGA, EQ-5D, and Piper fatigue scale). (Fig. 3). LSM [95% confidence interval] for PGA were: baseline: 66.4 [61.72–71.02] points, week 1: 71.8 [66.98–76.53] points, and for week 4: 73.8 [68.88–78.66] points (p=0.1 for baseline vs week 1 and p=0.018 for baseline vs week 4). For EQ-5D the LSM values were, baseline: 0.75 [0.70–0.79] points, week 1: 0.78 [0.73–0.83] points and week 4: 0.8 [0.75–0.85] points (p=0.2 for baseline vs week 1 and p=0.04 for baseline vs week 4). Finally there were significant decreases of the Piper fatigue scale at week 4. The LSM values were baseline: 4.5 [4.02–4.90], week 1: 3.9 [3.51–4.42] and week 4: 3.6 [3.19–4.13] (p=0.12 for baseline vs week 1 and p<0.008 for baseline vs week 4).
Exploratory variablesRaw data for laboratory parameters showed a significant increase in ferritin and TSAT at week 1 and week 4. (Table 2 Supplementary material). The inferential analysis is shown in Table 2. As expected, there was a significant increase of the iron status parameters, but not in hemoglobin levels or in eGFR.
Laboratory test. Inferential analysis of adjusted data.
| Variables | Baseline | Week 1 | p value baseline vs week 1 | Week 4 | p value baseline vs week 4 |
|---|---|---|---|---|---|
| Red blood cells, ×109/L | 3.89 [3.79–3.99] | 3.9 [3.81–4.0] | 0.9 | 3.64 [3.54–3.74] | 0.00 |
| Hb (g/dl)* | 10.97 [10.84–11.1] | 10.91 [10.78–11.05] | 0.46 | 11.07 [10.93–11.21] | 0.11 |
| Hematocrit, % | 36 [34.53–35.81] | 35 [33.95–35.34] | 0.47 | 34.1 [34.1–35.39] | 0.47 |
| Mean corpuscular volume, fL | 90.55 [88.93–92.17] | 90.95 [89.33–92.58] | 0.46 | 92.26 [90.63–93.9] | 0.00 |
| Mean corpuscular Hb, pg/cel | 28.43 [27.68–29.18] | 28.69 [27.94–29.44] | 0.03 | 29.28 [28.53–30.04] | 0.00 |
| Mean corpuscular hemoglobin concentration (g/dl) | 31.37 [31.02–31.72] | 31.64 [31.28–31.99] | 0.14 | 31.65 [31.28–32.01] | 0.15 |
| Leucocytes, ×109/L | 7.65 [6.95–8.35] | 7.39 [6.68–8.10] | 0.53 | 7.52 [6.8–8.23] | 0.85 |
| Platelets, ×109/L | 240.0 [217.46–262.64] | 232.0 [209.35–254.75] | 0.19 | 217.1 [194.35 239.96] | 0.00 |
| Urea, mg/dl | 79.90 [71.38–88.41] | 85.76 [77.22–94.30] | 0.07 | 85.02 [76.39–93.64] | 0.14 |
| Creatinine, mg/dl | 1.81 [1.73–1.88] | 1.80 [1.73–1.88] | 0.98 | 1.80 [1.73–1.88] | 0.95 |
| eGFR (CKD-EPI) (ml/min/1.73m2) | 33.52 [29.95–37.1] | 33.58 [29.98–37.18] | 0.99 | 33.82 [30.2–37.44] | 0.93 |
| Serum Iron (μg/dl) | 54.1 [45.5–62.54] | 96.5[87.71–105.22] | 0.00 | 74.8 [65.79–83.77] | 0.00 |
| TSAT (%) | 15.9 [13.19–18.75] | 31.1 [28.293–3.99] | 0.00 | 27.2[24.27–30.09] | 0.00 |
| Ferritin (ng/ml) | 48.5[6.13–90.95] | 618.7 [575.12 662.29] | 0.00 | 240.3 [195.64 285.155] | 0.00 |
| Phosphorus (mg/dl) | 3.74 [3.56–3.93] | 3.15 [2.97–3.34] | 0.00 | 3.56 [3.38–3.75] | 0.00 |
eGFR, estimated glomerular filtration rate; TSAT, transferrin saturation index.
The administration of IV FCM was well tolerated and no adverse events were reported. During the follow-up there were no deaths or hospital admissions.
We observed a significant (but assymptomatic) and transient drop in serum phosphorus levels at week 1 that partially recovered at week 4 (Table 2)
DiscussionThe main finding of the present study is the early and significant improvement in functional capacity, as measured by the 6-MWT, in patients with ND-CKD, mild anemia and ID after treatment with IV FCM, even in the absence of significant changes in hemoglobin levels. The improvement was seen early (1 week) and was maintained at 4 weeks. This finding suggest the deleterious effect of ID on the physical performance, independent of serum hemoglobin, in ND-CKD patients.21 The changes in the 6-MWT observed in our study were not only statistically, but also are clinically significant, according to previous studies.22,23 Furthermore, there was a significant improvement in the PGA, as well as in QoL and fatigue scores at 4 weeks, suggesting a deleterious role of ID, independent of anemia, in some PROMs in this population. These benefits can be attributed to the correction of ID due to its early effect (1 week) and since hemoglobin did not increase significantly from baseline. In fact, in a previous study in patients with CKD, the correction of anemia with ESA did not improve the 6MWT.24
ID is common in CKD.3,25 However, the deleterious effect of ID beyond anemia and whether it should be treated or not in CKD is a subject of increasing interest.8 Several studies have shown that ID in this population is associated with worse clinical outcomes9,10,26 as well as a poorer QoL,10 thus suggesting that ID should be considered for treatment independent of the presence of anemia in CKD patients, but confirming evidence from interventional studies is scarce.
The improvement in functional capacity with IV iron supplementation in our study agrees with previous results in ID patients with heart failure and reduced ejection fraction,12–14 as well with the improved aerobic capacity with iron therapy in ID athletes without anemia,27 suggesting a potential benefit on skeletal muscle energetics.28 Two randomized trials have been published which, similarly to our study, tried to demonstrate the benefit of IV iron therapy vs placebo on functional capacity in ND-CKD non-anemic ID patients. Both trials were placebo-controlled. However, both trials failed to show a benefit vs placebo in functional capacity. Bhandari et al. randomized 54 patients with a mean age of 59.6±11.7 years and a mean eGFR of 31.1ml/min/1.73m2 to receive 1000mg of ferric derisomaltose. The mean distance walked by the treatment group did not improve after treatment with IV iron at 1 and 3 months. The same authors acknowledge the limitations of the study, including an imbalance in baseline characteristics with a younger treatment group.15 Similarly, Greenwood et al. randomized 75 patients with ND-CKD mean age 57 years and mean eGFR was 35ml/min/1.73m2 to receive 1000mg of FCM or placebo. In this well conducted trial, there were no significant differences at 4 and 12 weeks, despite observing a numerical increase of 44 meters in the distance walked in the 6MWT in the group that received FCM vs 20m in the placebo group, this increase did not reach statistical significance.16 Both studies acknowledge that their patients had a good functional capacity at baseline.
The population included in both trials is certainly younger and in better physical condition than our population, as evidenced by the distance they covered at baseline in the 6MWT, which is significantly greater than in our group of older patients. In our opinion, our population more accurately represents the actual ND-CKD population with a mean age of 74.9±9.5 years, and a poorer physical condition, as demonstrated by the baseline distance walked in the 6MWT, which was around 100 meters less than the two mentioned studies.
We also aimed to analyze the impact of correcting ID on several PROMs. Utilizing tools for PROMs assessment is crucial for evaluating both the disease burden and the effectiveness of therapeutic interventions.
In the CKDopps study there was an association between ID and a poorer QoL, especially in the physical domain.11 In our study, we found an improvement in QoL at 4 weeks after adjustment, in contrast with the study of Bhandari et al.,15 but in agreement with the study of Agarwal et al.29
We also found significant improvements in the PGA and Pipper fatigue scale in our patients at 4 weeks after adjustment, suggesting the beneficial effect of ID correction on fatigue and well being, in contrast with the study of Greenwood et al.16
IV FCM was safe in our study, since no AE were reported and among patients that did not complete the study, reasons for discontinuation were not related to the study drug. There was a mild transient decrease in serum phosphorus levels at one week with partial recovery at four weeks, but no single patient experienced severe or clinical hypophosphatemia, as previously reported.30 The mean administered dose of IV FCM in our study (616mg) was calculated using the Ganzoni formula and is consistent with dosing strategies used in previous heart failure11 and nephrology trials, including the study by Greenwood et al. in ND-CKD patients, where a single FCM 1g dose was well tolerated and associated with functional improvements.16 Future studies should assess the benefits of simplified fixed-dose regimens (e.g., FCM 500mg single dose).
We acknowledge that the main limitation of this study is the absence of a placebo group. We opted for a single-arm study without a placebo group due to limited resources, and considering that the results in functional capacity (an objective measure) would be valid with this design. Furthermore, the present work is a pilot study. Our aim is, based on the current data, to conduct a randomized placebo-controlled study with a larger sample size in a ND-CKD population with these characteristics, since unlike previous trials, we observed significant benefits of the intervention in this older population with mild anemia and poorer physical status.
ConclusionsTo our knowledge, this is the first study to suggest the short-term benefit of IV FCM administration in improving physical function and other PROMs in elderly patients with CKD, ID, and mild anemia, despite no changes in serum in serum Hb levels, suggesting that the benefit was independent of anemia correction. Given the limitations of our study—small sample size, short time of follow-up, and absence of a control group—these findings should be considered as hypothesis-generating. Therefore adequately powered, randomized controlled trials with longer follow-up and the assessment of clinically relevant outcomes are needed to confirm these results.
FundingThis work has received an unrestricted grant from Vifor Pharma.
Conflict of interestMJP declares that have received speaker fees from Astellas, CSL Vifor and GSK.
AC declares that have received consultancy fees from AstraZeneca, Astellas, Boehringer Ingelheim, CSL Vifor, GSK, Lilly, Novonordisk and Otsuka and speaker fees from CSL Vifor, Astellas, Amgen, Bayer, Boehringer Ingelheim, Lilly, GSK, Novonordisk and Sanofi Mexico outside the submitted work.
JN has received consultancy and speaker fees and lectures from Astra Zeneca, Alleviant, Amgen, Bayer, Boehringer Ingelheim, CSL VIFOR, Daiichi Sankyo, GSK, Lilly, Pfizer, Novartis, Novonordisk, and Rovi.
JLG has been received consultancy fees from Astellas, GSK, CSL VIFOR, and speaker fees from AstraZeneca, Boehringer Ingelheim, Esteve, Bayer, Lilly, Astellas and Novonordisk.
JC, EGC, BG, MM, RDE, MGR, IT and CP declare no competing interest.
Data availability statementData are available on reasonable request.