The excessive chase for beauty standards and the rise of muscle dysmorphia have ultimately led to an increase in androgenic–anabolic steroids (AAS) and intramuscular injections of vitamins A, D and E (ADE) abuse, which is associated with several adverse effects and has become a public health issue. This review of literature discusses kidney injury associated with the use of AAS and ADE, highlighting the mechanisms of acute and chronic renal lesion, such as direct renal toxicity, glomerular hyperfiltration and hypercalcemia. Future perspectives regarding evaluation and early diagnosis of kidney injury in these patients are also discussed.
La búsqueda excesiva de los estándares estéticos y el aumento de casos de dismorfia muscular han llevado a un aumento excesivo del consumo de esteroides anabólicos androgénicos (AAS, por sus siglas en inglés) e inyecciones intramusculares de vitaminas A, D y E (ADE), que se asocian con varios efectos adversos y se convierte en un problema de salud pública. Esta revisión de literatura analiza la lesión renal asociada con el uso de AAS y vitaminas ADE, destacando los mecanismos de la lesión renal aguda y crónica, como la toxicidad renal directa, la hiperfiltración glomerular y la hipercalcemia. También se discuten las perspectivas futuras con respecto a la evaluación y el diagnóstico temprano de lesión renal en estos pacientes.
The excessive chase for beauty standards has contributed to the rise of a body image disorder, especially in the young male population: muscle dysmorphia, also known as bigorexia, which is characterized by an obsessive preoccupation that the own body is still insufficiently muscular.1,2 It is associated with other psychiatric conditions, such as mood disorders and anxiety1,3,4 and has ultimately led to an increase in androgenic–anabolic steroids (AAS) and intramuscular injections of oily compounds abuse.1,5
Steroid abuse is not restricted to professional bodybuilders. In fact, amateurs represent the largest share of AAS users.6 A recent study estimated that 2.9–4.0 million North Americans aged from 13 to 50 years had already used AAS, 32.5% of which developed dependence.7 These statistics reveal AAS dependence as a public health issue, with figures comparable to those of HIV infection and type-1 diabetes mellitus.8 In a Brazilian systematic review, the prevalence of AAS abuse was 2.1–31.6% and the highest prevalence was found among physical education professors and students. This finding is extremely alarming, for these individuals often help and guide other people's physical activity.9
Simultaneous use of multiple substances commonly impairs evaluation of adverse effects of each one in isolation.4 It is well known that AAS impacts several organ systems. Side effects vary from hypogonadism due to suppression of hypothalamus-pituitary-testis endocrine axis to cardiovascular disorders, including dyslipidemia, cardiomyopathies and arrhythmias.8,10,11 On the other hand, the abuse of intramuscular oily compounds (generally vitamins for veterinary use) might result in infection at the site of application, deformities, ulcerations, necrosis, embolism, nerve lesion and formation of foreign body granulomas, which isolate the injected material.5 Both substances are also associated with renal damage.
This review of literature discusses kidney injury associated with the use of AAS and intramuscular oily compounds, highlighting the mechanisms of renal lesion and future perspectives regarding evaluation and early diagnosis of kidney injury in these patients. It is important to note that the studies describing the adverse effects related to the use of such substances are still scarce, mostly due to ethical issues, poor understanding of the importance of this topic and patient's refusal to reveal their condition to physicians.8
Kidney injury associated with intramuscular vitamin supplementationVeterinary medications for bovines and equines containing vitamins A, D and E in an oily vehicle (known as ADE) do not enhance muscle hypertrophy, but the substance itself occupies space in the muscle surface or in the subcutaneous tissue, which leads to a result that is only visually similar to hypertrophy.5 While doses of ADE for veterinary purposes are, in average, 5mL every 120 days, in periods of fattening,5 Libório et al.12 reported a patient who used monthly doses of 50mL for 2 years.
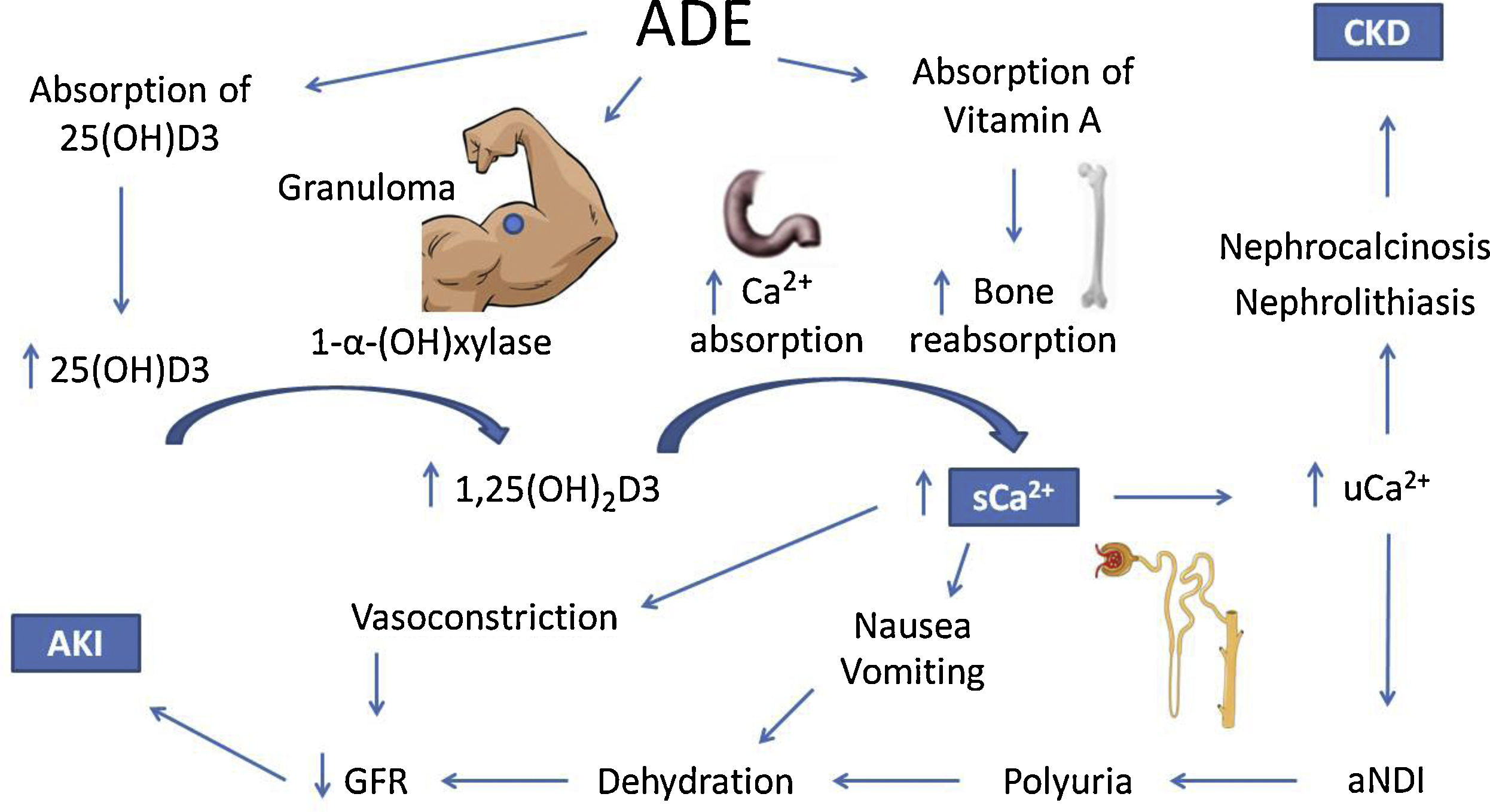
Both acute kidney injury (AKI) and chronic kidney disease (CKD) have been reported.12–17 A series of 16 ADE users by Daher et al.,18 revealed that 13 patients had AKI according to KDIGO,19 7 of which were stage III, 2 required dialysis, and one of them progressed to dialysis-dependent end-stage CKD. Other important findings were hypercalcemia (Ca2+ >10.5mg/dL or 2.62mmol/L) in 11 patients; high 25-hydroxivitamin D levels (>100ng/mL) in 9; and suppressed serum levels of parathyroid hormone (PTH)<10pg/mL in 6 (only 7 patients had their PTH assessed). Treatment consisted in controlling calcium levels and complications, mainly with vigorous crystalloid infusion, corticoids and loop diuretics. Following reversal of hypercalcemia, renal function tended to improve. The data are compatible with different reports in literature.12–17
The 25-hydroxivitamin D contained in the intramuscular injections is probably not the only responsible for the clinical picture, since the latent interval between the beginning of ADE use and hospitalization due to the onset of symptoms was 28 months,18 which is longer than expected. On the other hand, hypercalcemia also develops with cosmetic injections, like paraffin, silicone and polymethyl methacrylate.20 A possible common mechanism is the formation of granulomas, which express the 1-α-hydroxylase enzyme, which catalyzes the conversion of 25-hydroxivitamin D into its active form 1.25-hydroxivitamin D.21 This mechanism is corroborated by the decrease in calcium levels with the administration of glucocorticoids, which are effective against hypercalcemia associated with granulomatous diseases.22,23
However, a review by Tachamo et al.,20 analyzing 23 silicone and polymethyl methacrylate users, revealed a mean latent interval of approximately 95 months, which is even longer than that of ADE users. In addition, Tachamo et al.20 found high 1,25-hydroxivitamin D with low or normal 25-hydroxivitamin D levels, while Daher et al.18 found high levels of both. Therefore, the absorption of the 25-hydroxivitamin D contained in the injections is probably significant and may accelerate the onset of symptoms.
Although rare, hypervitaminosis A secondary to the absorption of the injected vitamin may also contribute to hypercalcemia,24 possibly by increasing bone reabsorption via vitamin A receptors in osteoclasts.25–27 However, only one case report assessed vitamin A levels, which were on the upper limit of normality.17
Renal impairment secondary to hypercalcemia may happen through different mechanisms, such as reversible vasoconstriction due to calcium's direct effect on vascular smooth muscle, decreasing renal blood flow and leading to prerenal AKI.28 Acquired nephrogenic diabetes insipidus and polyuria are found in up to 20% of patients with persistent hypercalcemia and may also contribute to prerenal AKI. Pathophysiology is not completely elucidated, but impairment of the countercurrent mechanism and downregulation of aquaporins 2 and 3 may be involved.29–32 It is important to consider that polyuria might represent an adaptive mechanism, for dilution of urinary calcium decreases chances of precipitation in renal tissue.
Chronic hypercalciuria originates Randall plaques, which are calcium-phosphate precipitates on the basement membrane of the thin descending limb of the loop of Henle. These plaques grow towards the tubular lumen or the interstitium, causing nephrolithiasis or nephrocalcinosis, respectively, by serving as anchored sites for calcium-oxalate adhesion.33–36 These effects are responsible for the progression to CKD.18
The mechanisms of hypercalcemia and their association with kidney injury in ADE users are illustrated in Fig. 1.
Possible mechanisms of hypercalcemia and kidney injury associated with the use of intramuscular vitamins A, D and E. ADE, intramuscular vitamins A, D and E. 25(OH)D3, 25-hydroxivitamin D. 1,25(OH)2D3, 1, 25-hydroxivitamin D. sCa2+, serum calcium. uCa2+, urinary calcium. aNDI, acquired nephrogenic diabetes insipidus. CKD, chronic kidney injury. AKI, acute kidney injury.
Testosterone and its synthetic hormonal derivates are collectively called AAS, given their anabolic and androgenic effects. More studies are being performed in order to develop substances with predominant anabolic action.8,37,38 Such substances would reduce adverse effects such as testicular atrophy, infertility, erectile dysfunction, gynecomastia and loss of libido.8
In the United States, the most frequently used AAS by high performance athletes, are testosterone, stanozolol, nandrolone and boldenone.8 Among amateur bodybuilders, boldenone, nandrolone, trenbolone and testosterone are the most common.7 A study performed by Iriart et al.39 in Northeast Brazil indicated testosterone and nandrolone as the most often used AAS.
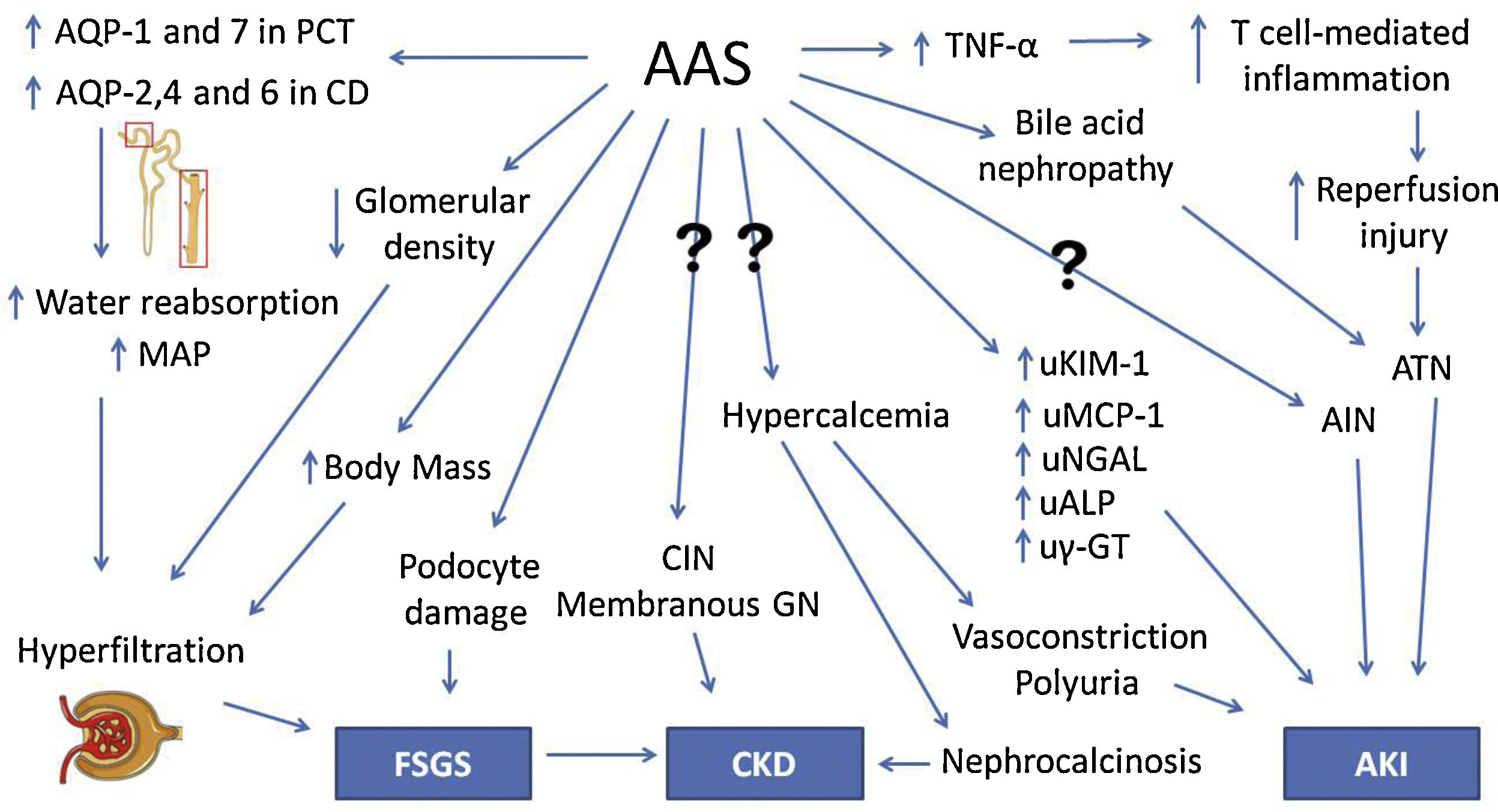
A six-year follow-up study by El-Reshaid et al.40 analyzes renal biopsies of 22 AAS and growth hormone (GH) users with high-protein dietary intake who were referred to the local nephrology unit due to altered renal function, hematuria and/or proteinuria. Focal segmental glomerulosclerosis (FSGS) was found in 8 patients, nephroangiosclerosis in 4; chronic interstitial nephritis (CIN) in 3; acute interstitial nephritis (AIN) in 2; nephrocalcinosis with CIN in 2; membranous glomerulopathy in 1; crescentic glomerulopathy in 1; and sclerosing glomerulonephritis in 1. All patients with nephrocalcinosis were hypercalcemic and reported high consumption of milk (in average, 10l per day) instead of water, in order to increase protein intake. During the follow-up, it was observed that patients diagnosed with FSGS had longer exposure to AAS, later disease onset and worse prognosis. On the other hand, patients with interstitial disease, as CIN or AIN, had shorter exposure to AAS and earlier disease onset, with stabilization or improvement after withholding AAS.40
Herlitz et al.41 studied renal biopsies of 10 AAS users who presented proteinuria and renal failure. Nine patients were diagnosed with FSGS. Direct glomerular toxicity by AAS and glomerular hyperfiltration due to increased body mass (similarly to obesity) were proposed as mechanisms of injury. Doublier et al.42 conducted experimental studies with female estrogen receptor knockout mice and also demonstrated the development of glomerulosclerosis. It was concluded that androgen and estrogen receptors in podocytes have opposite effects: the former induces damage and apoptosis while the latter has a protective effect.
Satoshi et al.43 have performed an experiment with orchidectomized rats that suggested a testosterone-mediated increase in urinary leucine aminopeptidase (LAP), γ-glutamyl transpeptidase (γ-GT) and alkaline phosphatase (ALP), which are enzymes present on the brush border membrane (BBM). Although also present elsewhere in the human body, they are too large to undergo glomerular filtration, hence their presence in the urine indicates renal damage or their upregulation on the BBM. Urinary glucose, cystatin C and β2-microglobulin, which are reabsorbed by renal tubules, were also increased by testosterone.
Another experimental study by Patil et al.44 with male rats found a protective effect of low-dose testosterone against ischemia-reperfusion injury. However, a higher dose increased TNF-α and intrarenal T cells, failing to elicit a protective effect. Therefore, TNF-α is possibly a key cytokine involved in AAS-associated renal damage. Loh et al.45 demonstrated testosterone-mediated increased expression of aquaporins (AQPs)-1 and 7 in the proximal convoluted tubule (PCT) and AQPs-2,4 and 6 in the collecting duct (CD) and consequent elevation of mean arterial pressure (MAP) in rats. This finding suggests that AAS could increase water reabsorption, causing hypertension and chronic kidney disease.
Urological changes may also be involved in AAS renal damage. Shortliffe et al.46 observed an increased bladder-to-body mass ratio and a lower collagen-to-smooth muscle ration in the bladder of rats treated with testosterone. Kidney-to-body mass ratio was also increased, although there was a lower glomerular density in the group treated with testosterone.
In order to increase bioavailability and enable oral administration, 17-α-alkylation of AAS molecules has been performed. Stanozolol, one of the most frequently used AAS, is an example of a 17-α-alkylated androgen. However, these steroids are known to be hepatotoxic and cases of severe cholestasis associated with AKI have been reported.47,48 Bile acid nephropathy is thought to be the pathophysiologic mechanism of renal impairment in these patients.47 Besides hepatic tumors, such as adenoma, which is well associated with AAS, there are also isolated reports of Wilms tumor.49 In addition, hypercalcemia and nephrocalcinosis in AAS users who had not received intramuscular oily solutions have also been reported.50,51 The underlying pathophysiology in these patients is still elusive.52–54
An experimental study with rats conducted by Frankenfeld et al.55 revealed disruption in redox homeostasis in the liver, heart and kidneys of animals treated with AAS. Chronic kidney disease in AAS users is usually asymptomatic for a long time before diagnosis. Moreover, given their increased muscle mass, creatinine levels are frequently elevated in this group of patients, even when renal damage is absent. Therefore, new strategies are needed for accurate and timely diagnosis, and novel biomarkers are currently being studied for that purpose. Daher et al.56 conducted a recent study and demonstrated an increase in creatinine and monocyte chemoattractant protein-1 (MCP-1) levels among AAS users, suggesting subclinical renal inflammation. It was our group's first study to analyze the role of novel biomarkers in AAS-related kidney injury. The pathophysiology of renal damage due to AAS use is summarized in Fig. 2.
Pathophysiology of kidney injury due to AAS use. AAS, anabolic androgenic steroids. Question marks indicate mechanisms that are not understood. AQP, aquaporin. PCT, proximal convoluted tubule. CD, collecting duct. MAP, mean arterial pressure. FSGS, focal segmental glomerulosclerosis. TNF-α, tumor necrosis factor α. ATN, acute tubular necrosis. AIN, acute interstitial nephritis. CIN, chronic interstitial nephritis. GN, glomerulonephropathy. uKIM-1, urinary kidney injury molecule 1. uMCP-1, urinary monocyte chemoattractant protein 1. uNGAL, urinary neutrophil gelatinase-associated lipocalin. uALP, urinary alkaline phosphatase. uγ-GT, urinary γ-glutamil transpeptidase. CKD, chronic kidney disease. AKI, acute kidney injury.
The inadvisable use of AAS and parenteral vitamins is a public health issue. Parenteral ADE is well associated with vitamin D overload and hypercalcemia, which probably derives from 1-α-hydroxylase-producing granulomas as well as absorption of the injected vitamin D. Hypercalcemia leads to nephrocalcinosis, nephrolithiasis and prerenal AKI. In AAS abuse, glomerular and interstitial damage occur by direct renal toxicity, glomerular hyperfiltration and hypercalcemia. Novel biomarkers for early diagnosis are currently being studied. High levels of MCP-1 were associated with AAS use. Larger studies are needed, since the majority of evidence is based on case and series reports. Finally, raising awareness of the risks and the great health burden of AAS and ADE abuse is probably the best strategy available at the moment for preventing complications.
Key points- •
Steroid and vitamin supplements abuse is a public health problem that can cause kidney injury, including acute kidney injury and chronic kidney disease.
- •
The most frequent and severe complication associated with vitamin supplement abuse is related with extreme doses of vitamin D, which leads to hypercalcemia, calcifications and renal damage.
- •
Anabolic steroids use has been associated with glomerular abnormalities and proteinuria.
- •
Novel biomarkers could be important to early detect kidney injury associated with the use of these substances and prevent further injury.
The authors declare no conflicts of interest.
We are very grateful for the technical support provided for the development of this revision. E.F. Daher, G.B. Silva Junior and A.M.C Martins are recipients of a grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).