Losartan is widely used in the treatment of chronic kidney disease (CKD) and has achieved good clinical efficacy, but its exact mechanism is not clear. We performed high-throughput sequencing (HTS) technology to screen the potential target of losartan in treating CKD. According to the HTS results, we found that the tumor necrosis factor (TNF) signal pathway was enriched. Therefore, we conducted in vivo and in vitro experiments to verify it. We found that TNF signal pathway was activated in both unilateral ureteral obstruction (UUO) rats and human proximal renal tubular epithelial cells (HK-2) treated with transforming growth factor-β1 (TGF-β1), while losartan can significantly inhibit TNF signal pathway as well as the expression of fibrosis related genes (such as COL-1, α-SMA and Vimentin). These data suggest that losartan may ameliorate renal fibrosis through modulating the TNF pathway.
El Losartán es ampliamente utilizado en el tratamiento de la enfermedad renal crónica (CKD) y ha logrado buenos resultados clínicos, pero su mecanismo exacto aún no está claro. Utilizamos la técnica de secuenciación de alto rendimiento (HTS) para detectar posibles dianas de losartán para el tratamiento de la CKD. Según los resultados de HTS, encontramos un enriquecimiento de la vía de señalización del factor de necrosis tumoral (TNF). Así, realizamos experimentos in vivo e in vitro para verificar esto. Encontramos que, tanto en ratas con obstrucción ureteral unilateral (uuo) como en células epiteliales tubulares renales proximal humanas (HK-2) tratadas con factor de crecimiento transformador β1 (TGF-β1), se activó la vía de señalización del TNF. El losartán inhibe significativamente la expresión de las vías de señalización del TNF y genes relacionados con la fibrosis, como COL-1, α-SMA y vicentin. Estos datos sugieren que el losartán puede mejorar la fibrosis renal regulando la vía del TNF.
Chronic kidney disease (CKD), including chronic glomerulonephritis, IgA nephropathy and diabetic nephropathy, is a common chronic disease characterized by structural damage and functional decline of the kidney. The incidence of CKD has increased significantly in recent years, with a global prevalence of 10–15% and 10.8% in China,1,2 making it a major public health problem.
Renal fibrosis (RF) is a common pathological pathway for all types of CKD progressing to end-stage renal failure, which is an important indicator of the degree of decline in renal function and prognosis.3 It is the main goal of CKD treatment to delay the process of RF or even reverse it.
Previous studies have shown that many drugs have a delaying effect on renal fibrosis, for example angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blocker (ARB), statins, endothelin receptors, beta-blockers, and matrix metalloproteinase (MMP) inhibitors.4–9 Losartan, an angiotensin II (AG II) receptor antagonist (AIIA), is widely used in clinical and experimental studies, which has been used to treat CKD.10–12 It can block the effects of AG II in the circulation and local tissues to result in a strong and sustained reduction in blood pressure and a decrease in systolic and diastolic blood pressure by causing arteriolar vasoconstriction, sympathetic excitation and the increase sensitivity of pressure receptors.13 Losartan can also protect the kidney and delay the process of chronic renal insufficiency by improving renal hemodynamics, selectively dilating small glomerular arteries, lowering intra-glomerular pressure, reducing renal vascular resistance and proteinuria, increasing renal blood flow and glomerular filtration rate.14
However, its specific mechanism of action in patients with CKD is not unclear. In this study, we mainly investigated the regulatory mechanism of losartan against CKD based on the results of RNA sequencing.
Materials and methodsAnimal experiments30 Wistar male rats (age range, 7–8 weeks) were housed and maintained under a 12h light/12h dark cycle in the clean animal facility at Hebei University of Chinese Medicine. The animal experiment was approved by the Ethics Committee for Animal Experimentation of the Hebei University of Chinese Medicine (Hebei, China), approval number: DWLL2019001.
Rats were randomly divided into three groups: sham group, UUO group, UUO+ losartan group (LST group). The kidney injury was induced by unilateral ureteral obstruction (UUO).15 This experiment used UUO for 14 days to replicate an animal model of renal fibrosis. At this time, most of the renal tubules in the rats were damaged, and the proximal and distal tubules were structurally disordered, resulting in significant renal injury and collagen deposition. The fibrosis characteristics were significant, and this model was not affected by exogenous drugs or toxins, making it an ideal method for modeling renal fibrosis.16 The rats were anesthetized with isoflurane by continuous inhalation. UUO was performed under sterile conditions. In sham group, the left ureters underwent an identical procedure but without ligation. In UUO group, rats were subjected to unilateral ureteral ligation and received no treatment. Rats in LST group were administered with losartan (20mgkg−1).17 Two weeks after surgery, rats were sacrificed after urine collection, the left kidney tissues were collected for histological and protein analysis.
High-throughput sequencing technologyWeigh 50mg of the kidney tissues of sham group, UUO group and LST group respectively for homogenization and RNA extraction. The extracted RNA is separated and fragmented for mRNA, and then double stranded cDNA is synthesized. Then, purify the cDNA with magnetic beads, prepare the end repair reaction solution, and then carry out end repair. Connect the repaired cDNA to the sequencing connector, and the generated library is PCR amplified, purified, and inspected. The qualified sequencing library uses illumina NovaSeq 6000 high-throughput sequencing platform for online sequencing.
Cell cultureHuman proximal renal tubular epithelial cells (HK-2) were purchased from Pronosai Life Sciences (Wuhan, China). Cells were cultured in DMEM F12 containing 10% fetal bovine serum (FBS) (Cat#: 0025, Gibco) at 37°C in 5% CO2. HK-2 were treated with 10ng/ml TGF-β1 (Cat#: HZ-1011, Proteintech) for 24h to establish an in vitro renal fibrosis model with or without losartan (10−6molL) (Cat#: HY-17512, MCE) or etanercept (3μg/ml) (Sunshine Guojian Pharmaceutical Co., Ltd) intervention.
Histological analysis, immunohistochemistry, and immunofluorescence analysisThe kidney tissues were embedded in paraffin blocks after being fixed overnight in 4% paraformaldehyde (PFA). Paraffin blocks were cut into 6μm sections for hematoxylin–eosin (HE) staining, Masson staining, immunohistochemical and immunofluorescence.
HE staining was carried out to estimate the inflammatory cell infiltration and tubulointerstitial changes of renal tissues, Masson staining was conducted to evaluate collagen deposition. Immunofluorescence analysis was conducted to detect the expressions of Vimentin (1:100, Cat#: ab92547, abcam) and COL-1 (1:100, Cat#: ab270993, abcam). Immunohistochemical analysis was conducted to detect the expressions of TNF-α (1:100, Cat#: A0277, Affinity), NF-κB (1:100, Cat#: 10745-1-AP, Proteintech), IL-6 (1:100, Cat#: 21865-1-AP, Proteintech). Image analyses were carried out by Image J 6.0 software (US National Institutes of Health, Bethesda, MD, USA).
Western blotting analysisTo prepare protein extracts, the kidney tissues were homogenized and lysed in ice-cold RIPA buffer for 30min. Then the lysates were centrifuged at 8000rpm for 10min at 4°C to obtain protein extracts. Protein extracts from kidney tissues and cells were separated by SDS-PAGE and transferred to PVDF membrane. After blocking with 5% milk 1h at 37°C, the membranes were incubated with primary antibodies against GAPDH (1:3000, Cat#: 10494-1-AP, Proteintech), α-SMA (1:1000, Cat#: ab5694, abcam), Vimentin (1:1000, Cat#: ab92547, abcam), COL-1 (1:500, Cat#: ab270993, abcam), TNF-α (1:1000, Cat#: A0277, Affinity), NF-κB (1:1000, Cat#: 10745-1-AP, Proteintech), IL-6 (1:1000, Cat#: 21865-1-AP, Proteintech) at 4°C overnight. After incubating with HRP-conjugated respective secondary antibody (1:5000, Cat#: SA00001-2, Cat#: SA0001-1, Proteintech). The chemiluminescence imaging analyser (ImageQuant LAS 4000, USA) detects antibody-specific binding bands. Band intensities were quantified with Image J.
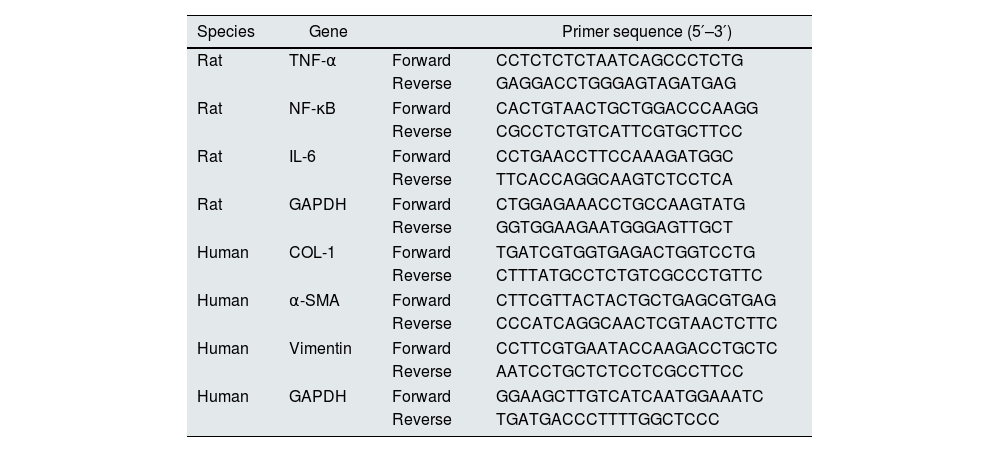
RNA isolation and quantitative reverse transcription-PCR (qRT-PCR)Total RNAs of cells and tissue samples were isolated using RNA extracting reagent (Omega Bio-Tek, Cat#: R6934-01). cDNA was transcribed from total RNA using SweScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (Servicebio, Cat#: G3337-50), according to the manufacturer's protocol. We conducted qRT-PCRs using SYBR Green qPCR Master Mix kit (High ROX, Servicebio, G3326-05). ABI 7500 FAST system (Applied Biosystems, Lincoln Center Drive, Foster City, CA, USA) was used to detect the mRNA level of related indicators. All primers were provided by Servicebio (Wuhan, China). Relative mRNA expression was normalized to GAPDH levels, using the 2−ΔΔCt method. The sequence for each primer was listed in Table 1.
The primers for qRT-PCR.
| Species | Gene | Primer sequence (5′–3′) | |
|---|---|---|---|
| Rat | TNF-α | Forward | CCTCTCTCTAATCAGCCCTCTG |
| Reverse | GAGGACCTGGGAGTAGATGAG | ||
| Rat | NF-κB | Forward | CACTGTAACTGCTGGACCCAAGG |
| Reverse | CGCCTCTGTCATTCGTGCTTCC | ||
| Rat | IL-6 | Forward | CCTGAACCTTCCAAAGATGGC |
| Reverse | TTCACCAGGCAAGTCTCCTCA | ||
| Rat | GAPDH | Forward | CTGGAGAAACCTGCCAAGTATG |
| Reverse | GGTGGAAGAATGGGAGTTGCT | ||
| Human | COL-1 | Forward | TGATCGTGGTGAGACTGGTCCTG |
| Reverse | CTTTATGCCTCTGTCGCCCTGTTC | ||
| Human | α-SMA | Forward | CTTCGTTACTACTGCTGAGCGTGAG |
| Reverse | CCCATCAGGCAACTCGTAACTCTTC | ||
| Human | Vimentin | Forward | CCTTCGTGAATACCAAGACCTGCTC |
| Reverse | AATCCTGCTCTCCTCGCCTTCC | ||
| Human | GAPDH | Forward | GGAAGCTTGTCATCAATGGAAATC |
| Reverse | TGATGACCCTTTTGGCTCCC | ||
Data analysis was performed with SPSS 26.0 and GraphPad Prism 8, and data were presented as the mean±standard deviation of the mean (SD). Comparisons between multiple groups were performed using one-way analysis of variance (ANOVA). P<0.05 was considered statistical significance.
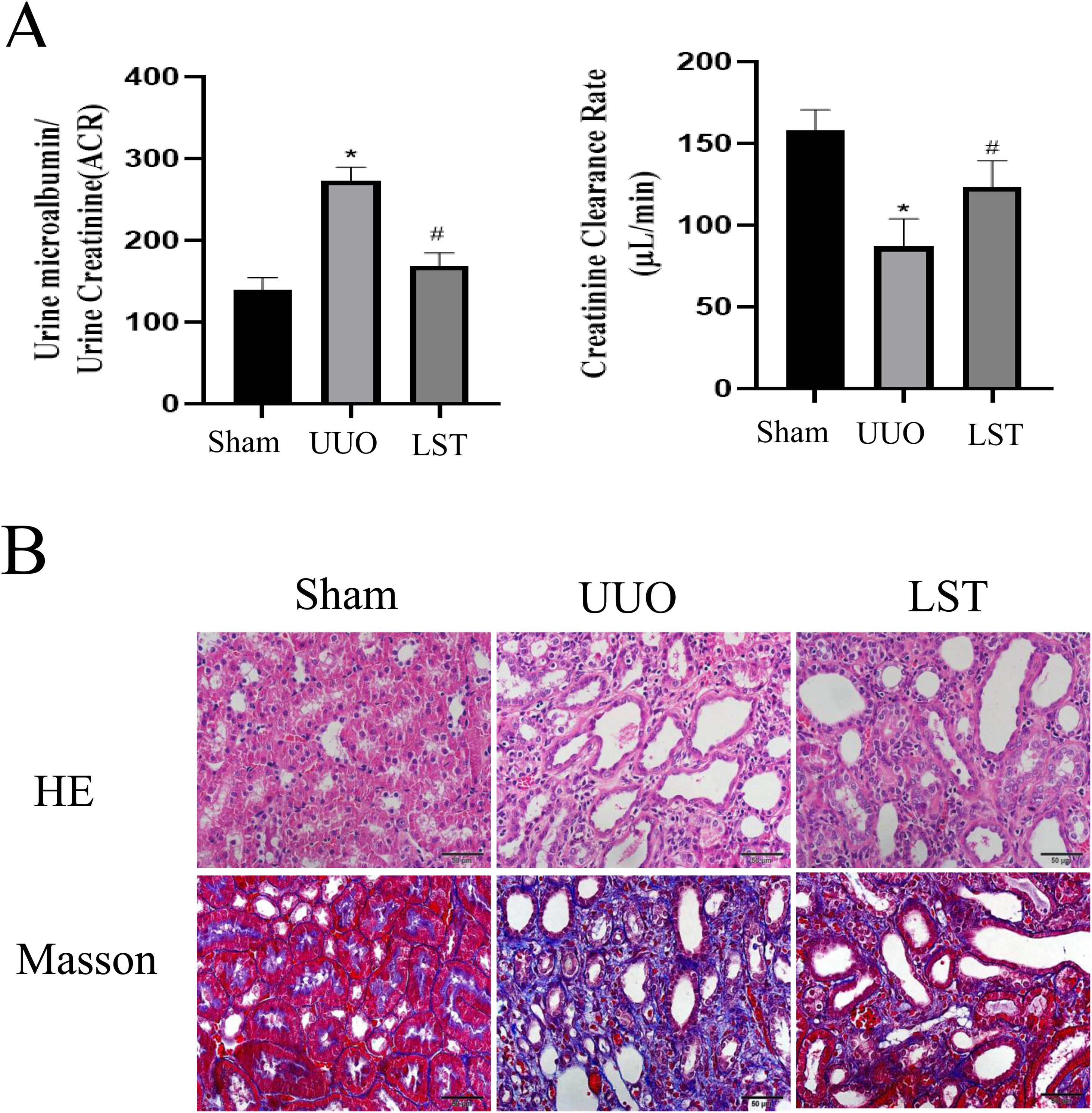
ResultsLosartan can alleviate renal pathological damage in UUO ratsAfter establishing UUO rats model, we first investigated the effects of losartan on renal function. The endogenous creatinine clearance (Ccr) rate and urine microalbumin to urinary creatinine ratio (ACR) were measured to assess renal function of rats. UUO rats showed significant renal damage compared with the sham group, and losartan could improve UUO-induced renal damage (Fig. 1A). HE staining was used to evaluate the degree of kidney damage in rats (Fig. 1B). In brief, the renal tissue structure of the sham group was normal. the renal tubule structure of rats in UUO group was severely damaged, with a large number of epithelial cells necrosis, thickening or disappearance of basement membrane, obvious proliferation of renal interstitial fibrous tissue, and infiltration of a large number of monocytes. Compared with UUO group, the above pathological damage was significantly reduced after losartan treatment. Masson staining was performed to evaluate the degree of renal fibrosis in rats (Fig. 1B). There was no significant fibrosis in the kidney tissues of the sham group. However, the renal cells were in disorder, the tubular structure was destroyed, and the collagen deposition was obviously increase in the model. In addition, the renal fibrosis in the LST group was reduced compared with UUO group. The above results indicate that losartan can alleviate renal pathological damage.
Losartan can alleviate renal pathological damage in UUO rats. (A) Endogenous creatinine clearance (Ccr) rate and urine microalbumin to urinary creatinine ratio (ACR). (B) HE was used to examine morphological changes and inflammatory cell infiltration. Masson was used to examine collagen deposition. The data are presented as the mean±SD, *P<0.05 vs. the Sham group, #P<0.05 vs. the UUO group. UUO: unilateral ureteral obstruction; LST: losartan.
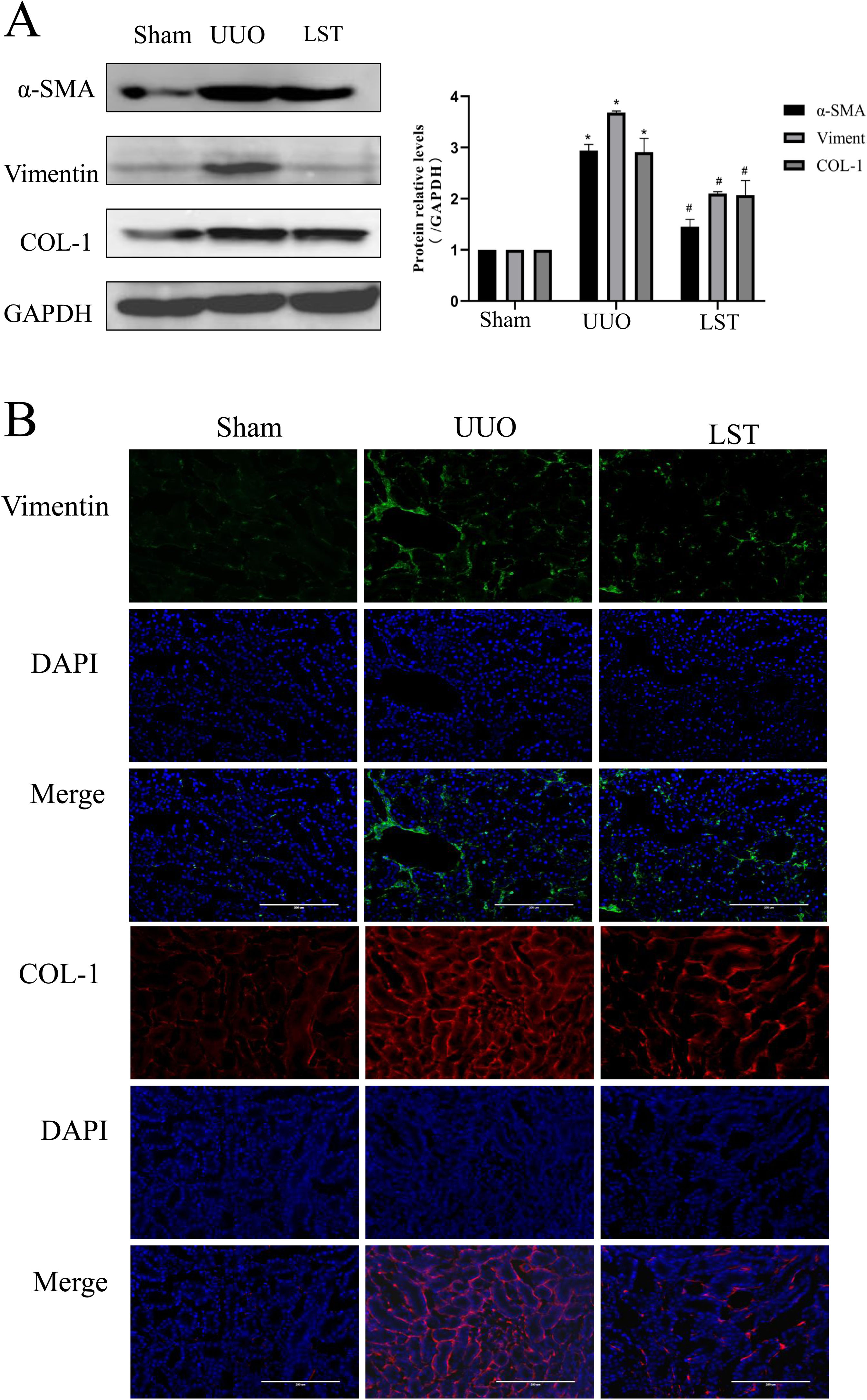
After evaluating the renal function and pathological damage of rats, we detected the changes of fibrosis indicators. The protein expressions of COL-1, α-SMA, and Vimentin were upregulated in the UUO group compared with the sham group, and losartan could decrease these protein expressions compared with the UUO group (Fig. 2A). We next assessed the protein levels of COL-1 and Vimentin in rat kidney tissues by immunofluorescence (IF). The results were in line with these of western blotting (Fig. 2B). Taken together, these results indicated that losartan can ameliorates renal fibrosis in UUO rats.
Losartan can ameliorate renal fibrosis in UUO rats. (A) Western blotting analysis using antibodies α-SMA, Vimentin and COL-1 against to examine renal fibrosis. (B) Immunofluorescence staining using antibodies against Vimentin and COL-1 to examine renal fibrosis. The data are presented as the mean±SD, *P<0.05 vs. the Sham group, #P<0.05 vs. the UUO group.
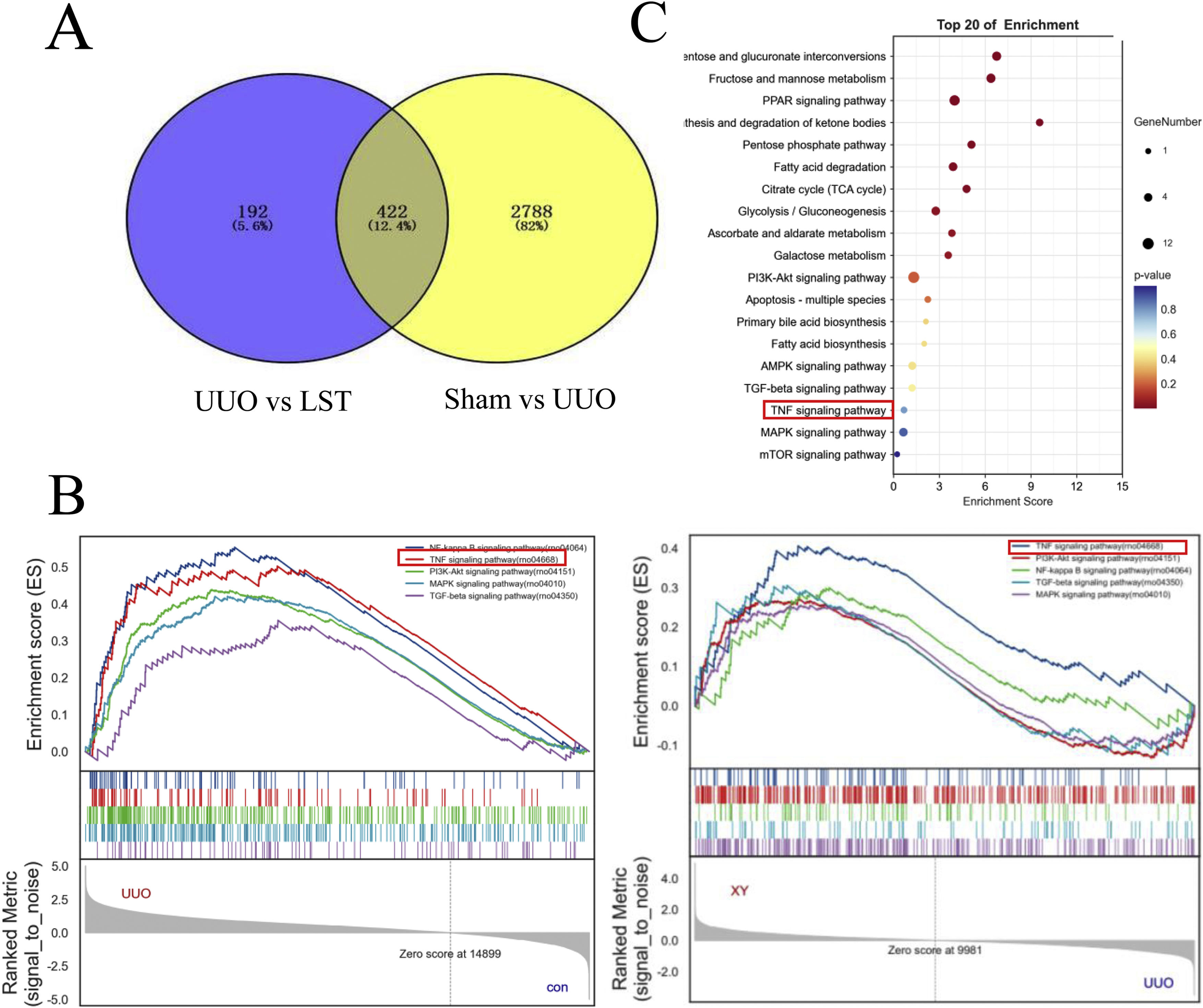
In order to further clarify the mechanism of losartan against renal fibrosis, we applied transcriptome sequencing (RNA-Seq) to screen the differentially expressed genes (DEGs) among the sham group, the UUO group and the LST group. Here, we used a comparative analysis of transcriptome analysis and gene expression. The results have shown that there were 3210 DEGs between the sham group and UUO group. Among them, it included 2395 up-regulated genes and down-regulated 815 genes in the UUO group by comparison with the sham group. Additionally, 614 DEGs were found between the LST group and UUO group. In which, 517 genes were up-regulated and 97 genes were down-regulated in the UUO group compared to the losartan group. We mapped the DEGs from the above two results to obtain 422 identical differential expressed genes, which might be the potential target genes for losartan against kidney fibrosis in UUO rats (Fig. 3A).
Losartan may ameliorate renal fibrosis through TNF signal pathway. (A) Venn Diagram of overlapping genes derived from transcriptome analysis in a pairwise comparison. LST vs. UUO in blue and Sham vs. UUO in yellow. (B) Gene set enrichment analysis (GSEA) of losartan treatment-related key pathways. (C) KEGG analysis of losartan treatment-related functional annotations.
To further discover the specific mechanism of losartan in delaying renal fibrosis, we conducted gene set enrichment analysis (GSEA analysis) with the above potential target genes. A total of 333 signaling pathways were enriched. As shown in Fig. 3B, TNF, TGF, MAPK and PI3K-Akt signaling pathways were the important signaling pathways. To identify the relevant pathways again, we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. The TNF pathway was also screened by KEGG enrichment results (Fig. 3C). Therefore, we mainly investigated TNF signaling pathway in the subsequent research.
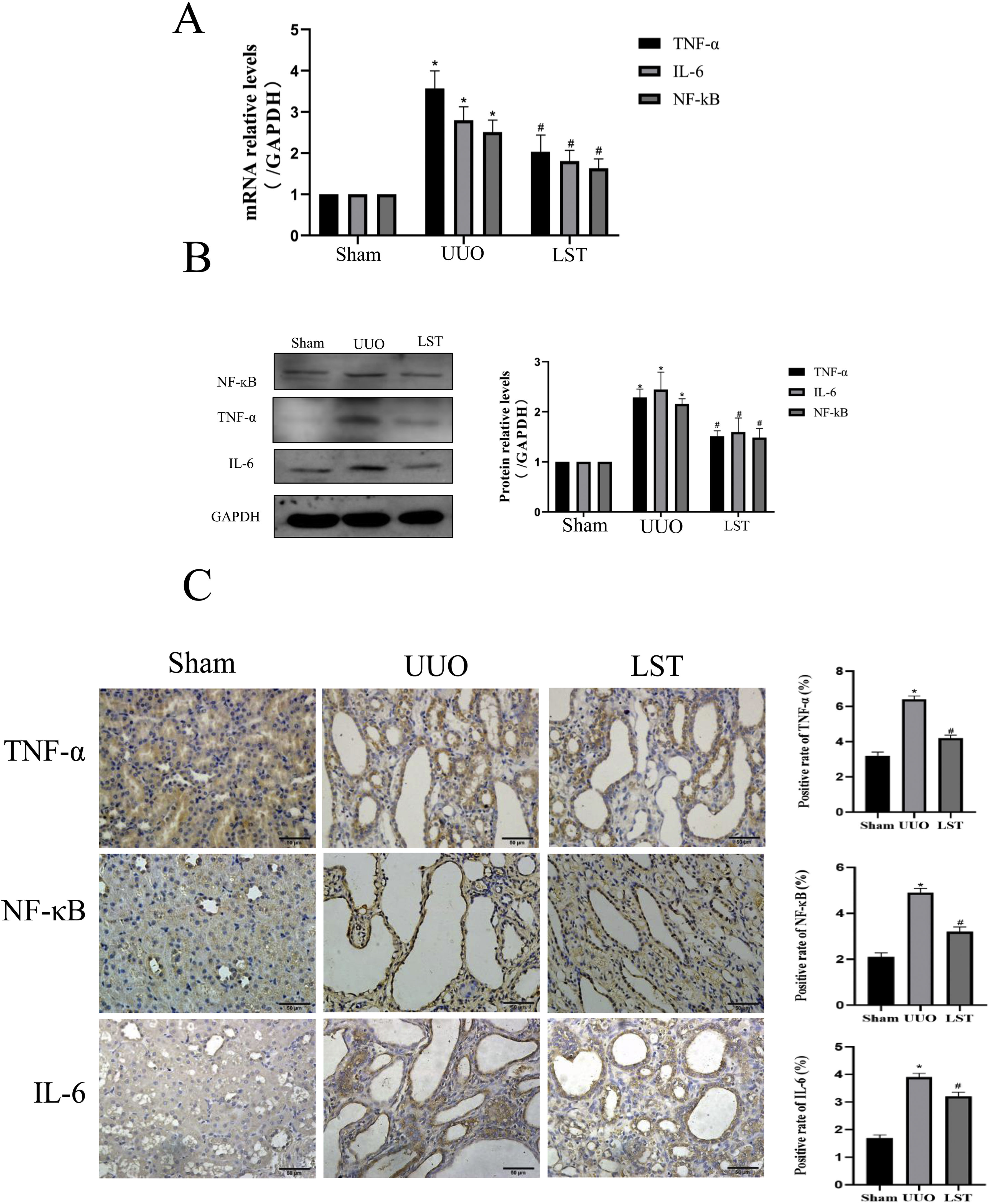
Losartan inhibits the expressions of genes related to TNF signal pathway in UUO ratsBased on the above results, we examined multiple genes involved in TNF signal pathway, such as TNF-α, IL-6, NF-κB. Fig. 4A shows that the mRNA expressions of these genes were higher in UUO group than sham group, while losartan could decrease those mRNA levels compared with the UUO group. In addition, we used western blotting to determine the protein levels of TNF-α, IL-6 and NF-κB, the results were the same as the PCR results (Fig. 4B). Immunohistochemistry results are consistent with PCR and western blotting (Fig. 4C). These results suggest that losartan can repress the expressions of genes related to TNF signal pathway.
Losartan inhibits TNF signal pathway expression in UUO rats. (A) PCR measured the mRNA expression of TNF-α, IL-6 and NF-κB. (B) Western blotting analysis using antibodies TNF-α, IL-6 and NF-κB against to examine inflammation. (C) Immunohistochemical analysis using antibodies TNF-α, IL-6 and NF-κB against to examine inflammation. The data are presented as the mean±SD, *P<0.05 vs. the Sham group, #P<0.05 vs. the UUO group.
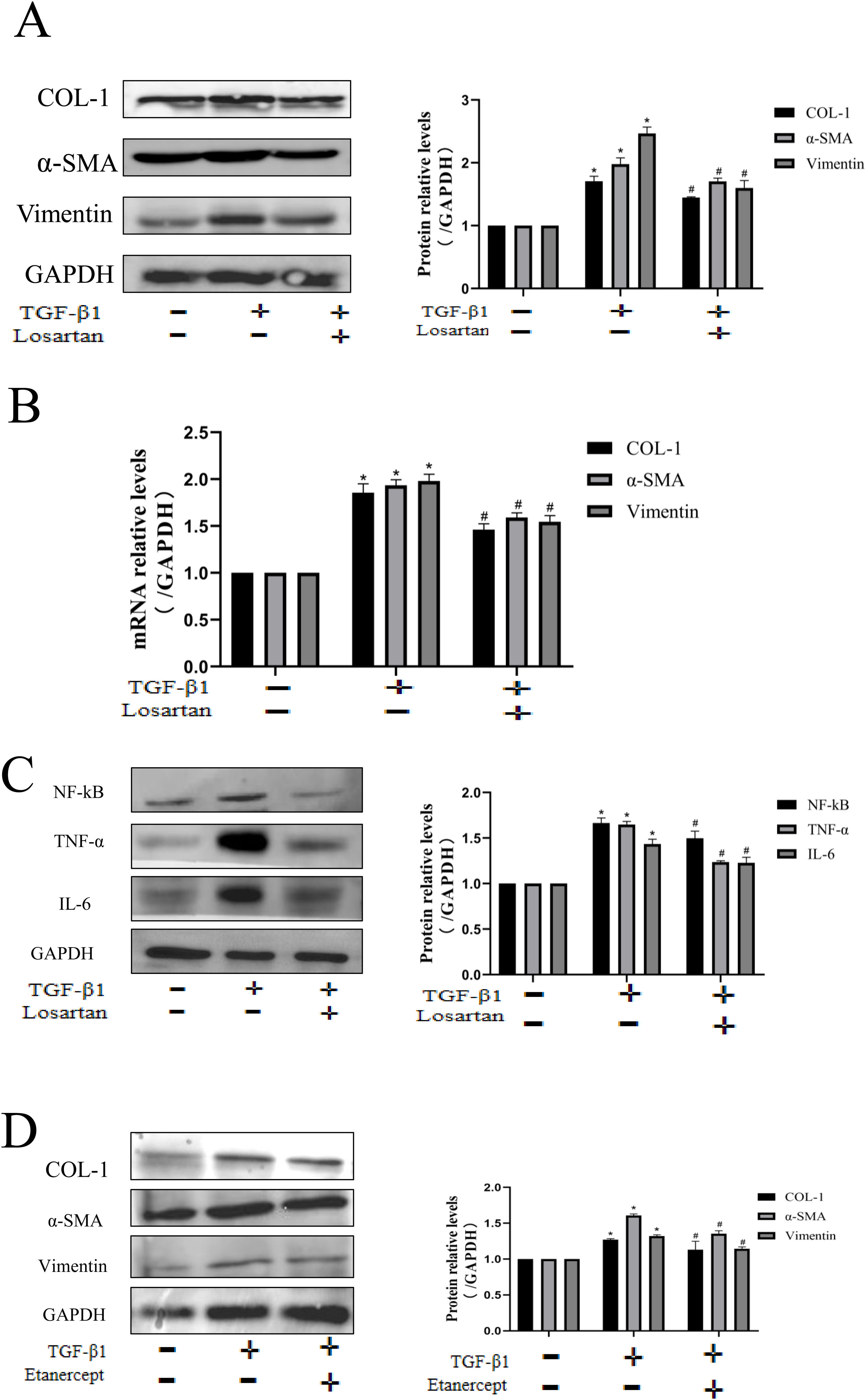
We used a TGF-β1 stimulated HK-2 to further verify the anti-fibrotic effect of losartan. The results showed that TGF-β1 could up-regulate the protein expression of COL-1, Vimentin and α-SMA, while the expression of these indicators was significantly inhibited by the intervention of losartan (Fig. 5A). The results of PCR were consistent with these of protein expression (Fig. 5B). Besides, in order to further clarify the mechanism of losartan against renal fibrosis, we examined the effect of losartan on regulating the TNF pathway in HK-2. The expression of TNF-α, IL-6 and NF-κB was significantly up-regulated after stimulation by TGF-β1, however, losartan could inhibit their increased expressions (Fig. 5C). Finally, we used TNF inhibitor (etanercept) to inhibit the activity of TNF in HK-2. As shown in Fig. 5D, etanercept could partially reverse the TGF-β1-induced increase of COL-1, Vimentin and α-SMA. These data confirm that losartan can inhibit TGF-β1-induced fibrosis by blocking the TNF pathway in vitro.
Losartan ameliorates renal fibrosis by TNF signaling pathway in HK-2 cell. (A) Western blotting analysis using antibodies α-SMA, Vimentin and COL-1 against to examine renal fibrosis. (B) PCR measured the mRNA expression of α-SMA, Vimentin and COL-1 to examine renal fibrosis. (C) Western blotting analysis using antibodies NF-κB, TNF-α and IL-6 to examine inflammation. (D) Western blotting analysis using antibodies α-SMA, Vimentin and COL-1 against to examine renal fibrosis. The data are presented as the mean±SD, *P<0.05 vs. the Con group, #P<0.05 vs. the TGF-β1 group.
The renin–angiotensin system (RAS) is important for the circulatory system and other organs.18–21 In recent years, the role of the renal RAS has become a hot topic of research. The renal RAS plays an important role in the regulation of fluid balance and long-term cardiovascular regulation. All the major components and key enzymes involved in the RAS, particularly the Ang II receptor subtypes AT1 and AT2, have been identified as being expressed in the kidney.22 Besides, contributing to or participating in systemic RAS function, the intrarenal RAS plays an important role in the regulation of renal function and the progression of renal disease.23–25
Losartan, also known as DuP753, is a non-peptide selective AT1 receptor blocker. Cloxacin was first discovered in 1992. Its chemical structure is 2-butyl-4-chloro-1-[(2′-(1H-etrazol-5-yl)(1,1-biphenyl-4-yl)methyl)]-1H-imidazole-5-methanol (C22H22N6O, 461.01).26 In addition to treating hypertension, losartan can also prevent the development of renal disease and other conditions in patients with type 2 diabetes.27 The long-term use of losartan or even other derivatives can further delay the progression of CKD which is dependent on their hemodynamic effects.28–30 But the exact mechanisms of losartan against CKD is currently unknown. Previous studies have shown that the delay of renal fibrosis by losartan may be closely related to inflammation, oxidative stress, cell proliferation and apoptosis, but its exact relationship is currently unknown.31–34
In this study, we evaluated the effect of losartan on improving renal fibrosis in both in vivo and in vitro experiments. The experimental results illustrated that losartan can significantly attenuate both UUO-induced and TGF-β1-induced renal fibrosis. To further investigate its molecular mechanism, we identified 422 mRNAs differentially expressed in LST group, UUO group and sham group. Subsequently, we conducted KEGG and GSEA pathway enrichment analysis. According to these results, there were mainly enriched four identical pathways, including PI3K-Akt, TGF, MAPK and TNF signaling pathway. The four signaling pathways are important in CKD, and their over-activation can trigger the development of CKD.35–37 The TNF signaling pathway is the most classical signaling pathway in the regulation of CKD. Consequently, we selected the TNF signaling pathway for validation here. Our study suggests that TNF signaling pathway activation is involved in the development of CKD. TNF-α is the most closely related to renal fibrosis in the TNF family, and NF-κB and IL-6 are important factors of TNF-α.38–40 In the present study, we mainly detect TNF-α, NF-κB and IL-6 expression levels. After losartan treatment, the protein expressions of these genes were reduced compared with that in UUO rats as well as in TGF-β1-induced HK-2 cells and the TNF inhibitor (etanercept) could improve TGF-β1-induced renal fibrosis. Therefore, we conclude that losartan is able to alleviate renal tissue injury by inhibiting the activation of the TNF signaling pathway.
The anti-renal fibrotic effect of losartan has been studied, but its mechanism of action has not been fully elucidated. In this study, we investigated the mechanism of losartan against RF from the perspective of transcriptomic analysis and experimental validation in vivo and in vitro. The results showed that losartan might ameliorate renal fibrosis by regulating the TNF signaling pathway. However, more further studies are required to elucidate that losartan really works by regulating the TNF signaling pathway. Firstly, clinical data and samples need to be studied to support our findings. Second, RF is a complex pathological process involving various mechanisms such as autophagy, oxidative stress, impaired energy metabolism and apoptosis.41–43 Therefore, the further elucidation is still needed for the specific mechanism of losartan against RF.
Ethical approvalThe animal experiments were approved by the Animal Ethics Committee of Hebei University of Chinese Medicine (no. DWLL2019001). The experiments involving animals were performed in line with the principles of Laboratory Animals of the National Institutes of Health.
Authors’ contributionsXT W and Y Z conceived and designed research. HS W, JZ L and F F performed experiments. HS W, JZ L, CC Z and LJ G performed cell experiments. HS W, Z W and Y Z analyzed data. HS W and JZ L wrote the manuscript. Y Z and XT W approved final version of manuscript.
FundingThis work was supported by a grant from Key Program of Hebei Natural Science Foundation of Traditional Chinese Medicine, H2022423351.
Natural Science Foundation of Hebei Province, H2021423006.
Hebei University of Chinese Medicine 2023 Postgraduate Innovation Funding Project, XCXZZBS2023002.
Competing interestsThe authors declare that they have no competing interests.