Dear Editor,
Congenital nephrotic syndrome of the Finnish type (CNF; OMIM#256300) is a rare autosomal recessive genetic kidney disease that develops in utero and is usually diagnosed before the age of 3 months of postnatal life.1-3 This disease is defined by massive proteinuria, consecutive hypoproteinemic edema and dyslipidemia from birth. Renal protein loss is furthermore accompanied by hypogammaglobulinemia predisposing these infants to bacterial infections such as peritonitis and respiratory infections, as well as thromboembolic complications that have repeatedly been observed.2,4 Kidney histology in this condition shows progressive mesangial sclerosis and capillary obliteration, and tubulointerstitial fibrosis. Many affected infants are prematurely, have low birth weight, and show poor statural growth and nutritional status.4
The gene that is causative for this condition, NPHS1 (OMIM*602716), encodes a trans-membrane cellular adhesion molecule named nephrin which is a crucial component of the glomerular slit diaphragm to maintain the size-selective filtration barrier.1,4
CNF is a progressive kidney disease and typically leads to end stage renal disease (ESRD) between 3 to 8 years of age. Renal transplants have a considerable risk of recurrence of the glomerular disease due to anti-nephrin antibodies observed in most of affected patients3, 4.
Herein, an Iranian boy with CNF is presented who carries a novel mutation in the NPHS1 gene.
A 45-day-old infant was admitted to the Children’ Medical Center Hospital, the Pediatrics Center of Excellence in Iran, with chief complaint of generalized edema. There was a family history of edema and proteinuria in his sibling who died in 2nd month of life without a clear diagnosis. Parents are first cousins consanguine.
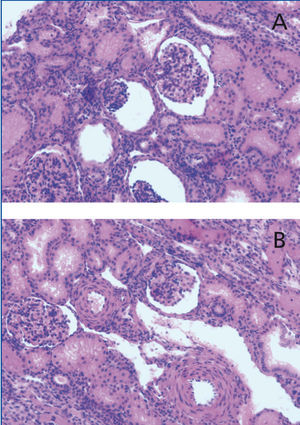
At physical examination, the patient had a weight of 4,750g, a body length of 52cm, and his blood pressure was 80/60mmHg. Laboratory data showed blood urea nitrogen (BUN) of 7mg/dL, creatinine (Cr) of 0.39mg/dL, total protein of 5g/dL, and albumin of 2.36g/dL. Other laboratory tests revealed the following values: cholesterol: 152mg/dL, triglyceridemia: 111mg/dL, calcium: 7.5mg/dL, P: 5.6mg/dL, alkaline phosphatase: 2,600IU/L, random protein/Cr: 525/44mg/mg and 3+ protein in urinalysis. TORCH study was negative and immunologic investigations were normal. Thyroid tests showed hypothyroidism (TSH: 38ng/dL, FT4: 0.4ng/L). By echocardiography, valvular pulmonary stenosis was seen. Kidney biopsy showed mesengial proliferation in glomeruli and proliferative changes in smooth muscle layer in the artery, suggestive for nephrotic syndrome (Figure 1). Therefore albumin infusions, levothyroxin, and enalapril (0.1mg/kg/d) and other supportive care were started for the patient.
He developed end-stage renal failure at the age of 6 years and received a renal allograft from an unrelated living donor. Four years later, he was hospitalized again with an abdominal mass. Burkitt’s lymphoma was diagnosed and he was put on chemotherapy. However, unfortunately, he died because of cancer progression 7 months later.
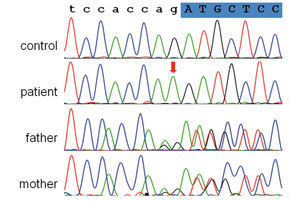
Genetic studies on DNA samples of the patient and his parents were performed to analyze the NPHS1 gene for suspected congenital nephrotic syndrome, Finnish type. Exons and flanking intronic regions of the gene (NPHS1 Exon 1-29, NM_004646) were amplified by PCR. PCR amplicons were purified and subjected to direct sequencing using an automated capillary sequencer. Sequences were compared to the reference sequences deposited in the public database (NCBI). In the patient, homozygosity for a 1 bp duplication near the splice acceptor site of exon 17 of the NPHS1 gene was detected (intron 16: c.2213-2dupA homozygous mutation). In both parents the heterozygous carrier status for the mutation was confirmed (Figure 2). This variation has not been described as a mutation or polymorphism, so far. It is predicted to abrogate splice acceptor function and lead to abnormal splicing of exon 17, thus indicating that this change is indeed a pathogenic mutation. These findings strongly confirmed the diagnosis of CNF in this patient.
The NPHS1 gene covers 26 kb of genomic sequence, comprises 29 exons, and is located on chromosome 19q13. It encodes nephrin, a single pass transmembrane protein consisting of eight extracellular immunoglobulin-like modules, a fibronectin type III-like motif and a cytosolic C-terminal tail. Nephrin represents an important structural and signaling protein in podocytes, that regulate ultrafiltration of proteins at the slit diaphragm and connects foot processes in a zipper-like fashion.5-8
Mutations in this gene cause an autosomal recessively inherited congenital nephrotic syndrome which was first diagnosed in Finland where its prevalence is particularly high due to a founder mutation effect.7 Detailed studies identified 2 main mutations in Finnish population, Fin major (L41fsX90) and Fin minor (R1109X), both leading to the absence of nephrin at the slit diaphragm structure. To date, many other NPHS1 mutations have been reported in Finnish and non-Finnish populations, including deletions, insertions, nonsense, missense, splice site and promoter mutations.5,7,8 We found a novel mutation in the presented case that also developed Burkitt’s lymphoma. Although it could be a rather a chance association, it has not been reported in the literature. Further studies on nature of this gene and follow-up of further cases with such mutation are needed to confirm this association.
Typical findings on electron microscopy include effacement of the foot processes of podocytes, villous formation and complete absence of the slit diaphragm.5,7 Renal histopathology usually shows mixed glomerular and tubulointerstitial lesions that vary from mesangial hypercellularity to glomerular sclerosis and/or diffuse mesangial sclerosis and progress with age. Affected individuals almost always develop nephrosis soon after birth and may already show prenatal signs of hypoproteniemia such as placental or skin edema.5,7 Fetal proteinuria leads to increase alpha-fetoprotein concentration in the amniotic fluid and a less important increase of it in maternal plasma, which has been used for prenatal testing especially in high-risk families.4,9 Affected infants are always resistant to steroids, immunosuppressive drugs and calcineurin inhibitors.4,7 These drugs may rather be harmful because of high susceptibility of these patients to infections.4 The disease is rapidly progressive and ESRD usually occur in the first decade of life.
Initial standard treatment for these patients is conservative, including daily or alternating albumin infusions, nutrition with a high-protein, low-salt diet, vitamin, thyroxine substitution and prevention of infection and thrombotic complications.4 Most of the patients require bilateral nephrectomy to prevent massive proteinuria before the development of renal failure. Dialysis is recommended until the patient’s weight reaches to 8-9kg. The only long term life-saving treatment is renal transplantation.5,7
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Histopathological findings in renal biopsy
Figure 2. Electropherograms showing the junction region of intron 16 and exon 17 (the shadow, uppercase letters) of the NPHS1 gene