Peritoneal dialysis (PD)-related infections due to nontuberculous Mycobacteria (NTM) are rare in children and adolescents but appear to be increasingly recognized. To better understand the clinical profile, characteristics, management and outcomes of these infections, we conducted a systematic review of pediatric cases reported in the literature. We identified 25 episodes in 23 patients under the age of 18, drawn from 19 studies. The mean age was 7.35 years with a slight male predominance. The most common underlying condition leading to end stage renal disease and initiation of PD, was congenital anomalies of the kidney and urinary tract, particularly hypoplastic or dysplastic kidneys. Clinical manifestations were non-specific, often including fever, abdominal pain and cloudy effluent. Among the reported cases 52% presented with peritonitis, 36% with exit site infection and 12% had both. Mycobacterium abscessus and Mycobacteriumfortuitum emerged as the most prevalent species, each accounting for a comparable proportion of the recovered isolates. Notably, Mycobacterium chelonae was the most frequent isolated specie among infants. Diagnosis was frequently delayed and treatment required prolonged antibiotic regimens. Catheter removal was performed in almost all cases, while most patients were temporarily switched to hemodialysis. Mortality rate was low approaching 4%. These findings underscore the need for heightened clinical suspicion, early diagnosis and aggressive management to improve outcomes in pediatric patients with PD-related infections due to NTM.
Las infecciones relacionadas con la diálisis peritoneal (DP) debidas a micobacterias no tuberculosas (MNT) son raras en niños y adolescentes, pero parecen ser cada vez más reconocidas. Con el fin de comprender mejor el perfil clínico, las características, el manejo y los desenlaces de estas infecciones, realizamos una revisión sistemática de los casos pediátricos reportados en la literatura. Se identificaron 25 episodios en 23 pacientes menores de 18 años, extraídos de 19 estudios. La edad media fue de 7,35 años, con un ligero predominio masculino. La condición subyacente más común que condujo a enfermedad renal terminal e inicio de DP fue la presencia de anomalías congénitas del riñón y de las vías urinarias, particularmente riñones hipoplásicos o displásicos. Las manifestaciones clínicas fueron inespecíficas, incluyendo con frecuencia fiebre, dolor abdominal y líquido efluente turbio. Entre los casos reportados, el 52% presentó peritonitis, el 36% infección del sitio de salida y el 12% ambas. Mycobacterium abscessus y Mycobacterium fortuitum surgieron como las especies más prevalentes, representando proporciones similares entre los aislamientos recuperados. Cabe destacar que Mycobacterium chelonae fue la especie más frecuentemente aislada entre los lactantes. El diagnóstico fue frecuentemente tardío y el tratamiento requirió regímenes antibióticos prolongados. En casi todos los casos se realizó la retirada del catéter, y la mayoría de los pacientes fue transferida temporalmente a hemodiálisis. La tasa de mortalidad fue baja, aproximándose al 4%. Estos hallazgos subrayan la necesidad de mantener una alta sospecha clínica, realizar un diagnóstico temprano y aplicar un manejo agresivo para mejorar los desenlaces en pacientes pediátricos con infecciones relacionadas con DP por MNT.
Peritoneal dialysis (PD)-related infections due to nontuberculous Mycobacteria (NTM) constitute a rare complication of PD, which is even rarer in children and adolescent, accounts less than 1% of peritonitis cases.1,2 Noteworthy is the fact that the incidence and prevalence rate of PD-related infections caused by NTM seems to have been increasing.3 The diagnostic problem for these specific infections based on the fact that can be misidentified as other pathogens including diphtheroids or Corynebacterium species, leading to delayed diagnosis.1 When suspected, there is a need for further evaluation requiring acid-fast bacilli stain and culture on specific media or automated system analysis even whole-genome sequencing (WGS). Species including Mycobacterium fortuitum, followed by Mycobacterium abscessus, Mycobacterium chelonae and Mycobacterium avium are the most common NTM isolates known to cause PD-related infections, both PD-related peritonitis or PD catheter-related infection such as exit-site infection (ESI) and tunnel infection.1,2,4 Problematic is also the appropriate management and treatment of these infections, especially with respect to the relocation of the exit site and catheter removal, which is remain unclear. However, in general two agents with in vitro activity against the clinical isolate of NTM infection for a minimum duration of 4 months, seems to be the appropriated treatment based on the ISPD recommendations.2
Data about the incidence, diagnostic methods, management, treatment and prognosis of NTM infections in children undergoing PD are scarce. It is noteworthy that there are no retrospective studies that include the full number of pediatric cases found in the literature, but usually include a smaller number of cases. Based on that, we conducted a thorough systematic review of literature focusing in PD-related infections caused by NTM in children and adolescents less than 18 years-old, because there are very limited data available. Accordingly, this literature review aimed to investigate in depth these rare and difficult to cure infections.
MethodsGeneral information and literature search strategyThis review conforms to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement.5,6 (PROSPERO 2024 CRD42024611361; available from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024611361; accessed on 18 November 2024).
Eligibility criteriaCase reports of PD-related infections, comprising peritonitis and/or exit site infections, due to NTM in children and adolescents, are incorporated in this review. The diagnosis of peritonitis was based on criteria covering clinical manifestations such as abdominal pain, nausea and fever combined with a cloudy peritoneal effluent count of 100 WBC/μL or greater, composing of at least 50% PMN cells and a positive culture of peritoneal effluent. With two at least of the above, diagnosis of peritonitis is established.2 An exit-site infection (ESI) was defined as the presence of purulent drainage, with or without erythema of the skin at the catheter-skin interface. In addition, tunnel infection may present as erythema, edema, or tenderness over the subcutaneous pathway but is commonly clinically occult.2,4 Tunnel infection usually occurs in the presence of an ESI. Both peritonitis and ESIs were defined as a case presenting with a positive culture for NTM from peritoneal fluid or pus or tissue culture from the exit site. The identification of NTM was made by acid-fast bacilli stain and established either by conventional tests or by an automated system [matrix-assisted laser-desorption ionization–time of flight mass spectrometry (MALDI–TOF)] or by dialysis effluent polymerase chain reaction (PCR) or by WGS.
Information sources and search strategyWe searched the literature for PD–related infections caused by NTM in children and adolescents (0 to ≤18 years-old). Articles were obtained from two databases: PubMed (US National Library of Medicine National Institutes of Health, www.ncbi.nlm.nih.gov/pubmed, accessed on 1 February 2025) and Google Scholar (Google, www.scholar.google.com, accessed on 1 February 2025). Databases were searched using the keywords “nontuberculous Mycobacteria” and “peritonitis” and/or “exit-site infection” and “peritoneal dialysis” and “children/adolescents” in any available language. For the translation of text, we used the Google online translation tool (Google, https://translate.google.gr, accessed on 28 February 2025).
Study selectionAll potentially relevant articles published through 1 February 2025 were screened in two stages for eligibility by selected authors based on their previous experience in such reviews. In the first stage of assessment, the titles and abstracts of potentially relevant articles were screened independently by three authors (JD, AK, AP). The reference list for each article was examined to verify that all published cases had been collected. For those abstracts that met the inclusion criteria, the full text was retrieved and independently reviewed by two authors in the second stage of assessment (VK, NP). Disagreements and technical uncertainties were discussed and resolved by all authors (JD, AK, VK, AP, NP).
Data extractionThe primary citations obtained during the database survey were recorded in a text file according to their topics and abstracts. None of the case reports found were excluded from enrolment in the analysis due to the quality or inadequacy of data reported, although in some reports all data were not available. Variables included in the database were the year of publication, origin, demographic information (gender and age), underlying disease leading to end stage renal disease (ESRD), previous peritonitis episodes, symptoms, temperature levels and laboratory findings during admission, predisposing factors, diagnosis method and NTM sensitivity patterns. In addition, data on treatment choice, duration and route of treatment, catheter removal procedure and outcome were also recorded.
Ethics statementDue to the retrospective, literature review nature of the study, the Ethics Committee of our Hospital, determined that patients’ consent was not required.
Statistical analysisAll articles found were systematically reviewed, and a master database was constructed. Microsoft Excel software (XP Professional) version 5.2.3790.1830 (Redmond, WA, USA) was used to develop this database of categorical and continuous variables. The statistical program Graphpad Instat 3.10 (Graphpad Inc., San Diego, CA, USA) was used. A two-sided p-value of <0.05 indicated statistical significance.
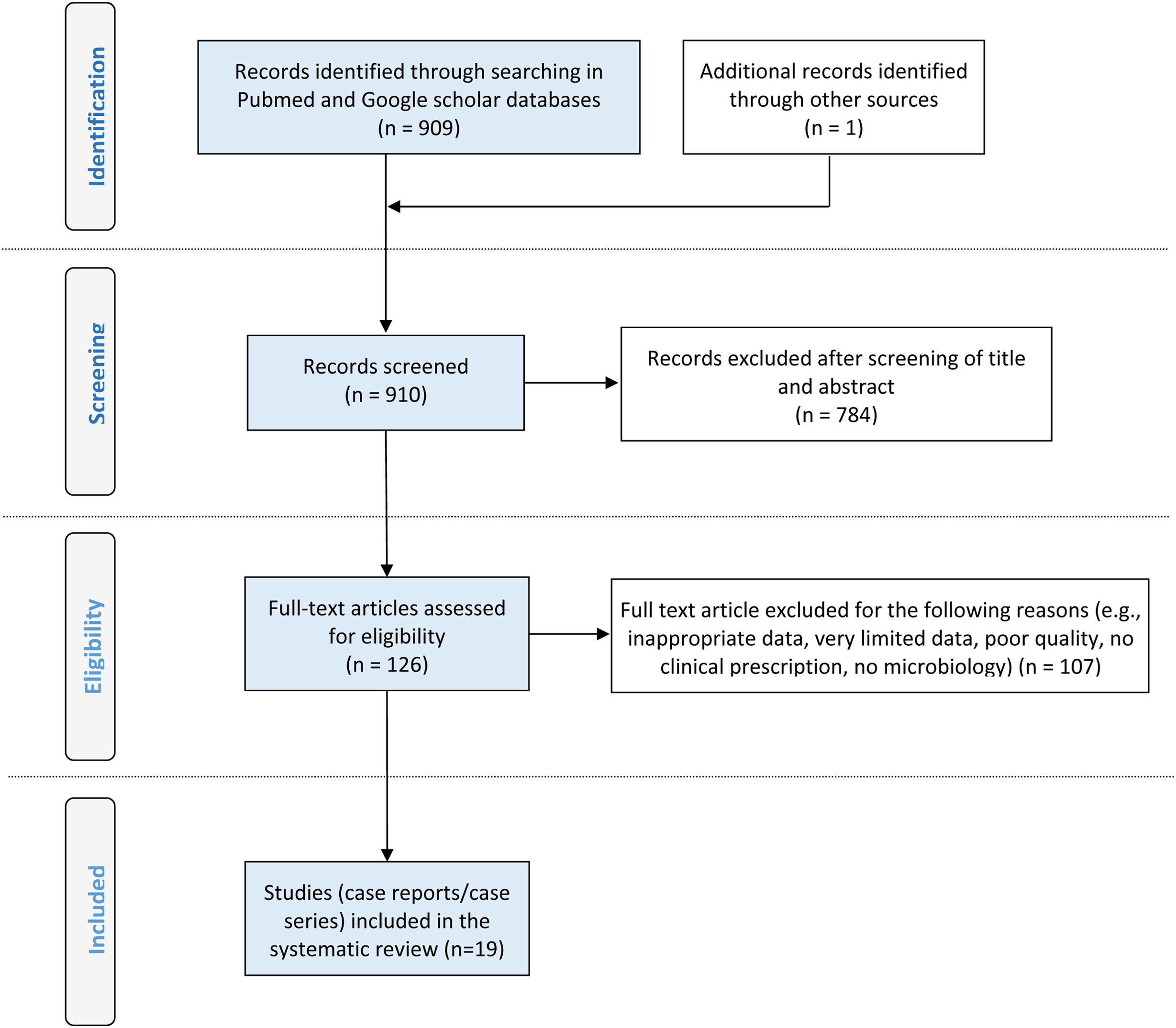
ResultsThe search strategy yielded 910 scientific articles which were reviewed. After selection using PRISMA methodology, a total of 25 cases of PD-related infections caused by NTM in children and adolescents were identified in 19 articles and presented in Fig. 1.7–25 The total number of cases involved 23 patients. The first report of NTM peritonitis in a PD child appeared in 1983.7 Their mean (±SE) age was 7.35±5.63 years (range, 3 months–18 years), while, the most cases occurred in males (15/25, 60%) (Table 1). Twelve (48%) of the cases were reported from Japan, followed by 7 (28%) which were from USA and 1 case each was from Qatar, Singapore, Spain, India, Canada and Saudi Arabia, respectively.
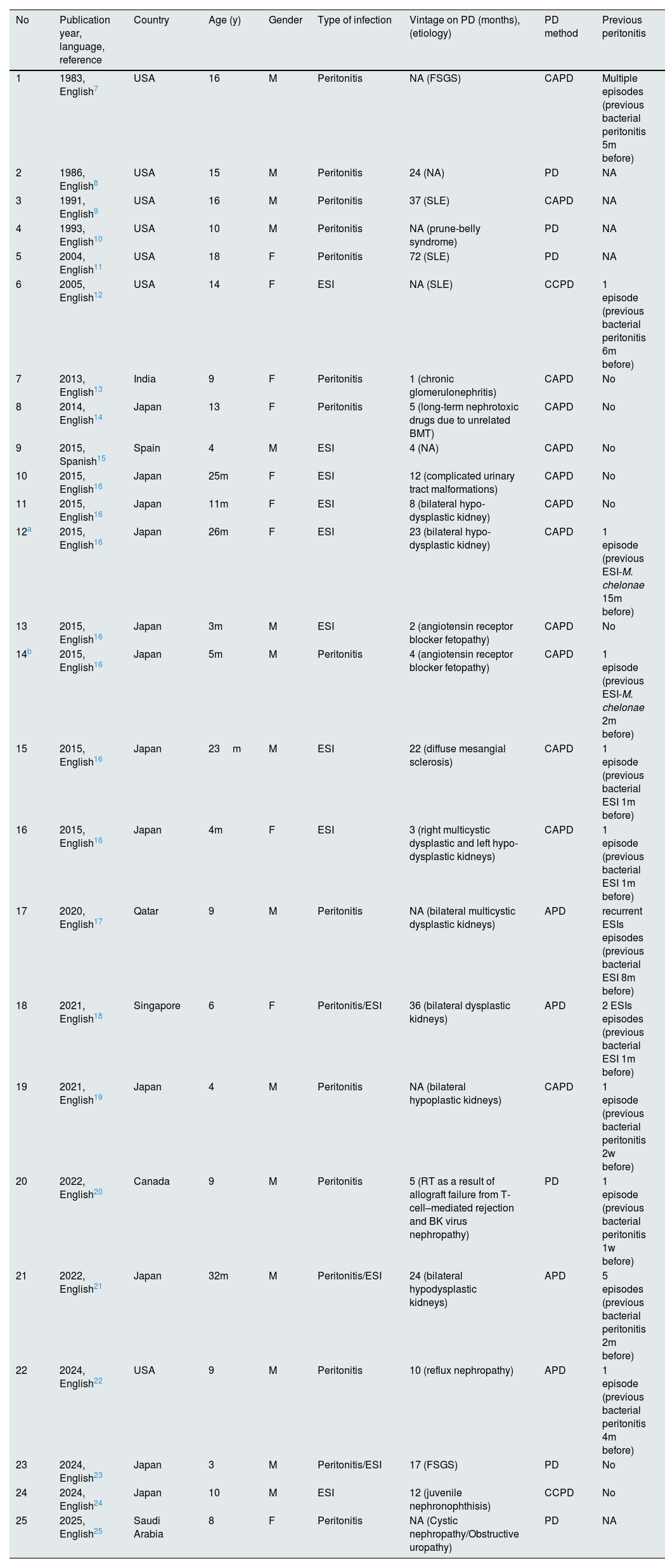
Cases of peritoneal dialysis-related infections due to nontuberculous Mycobacteria (sorting from older to newer).
| No | Publication year, language, reference | Country | Age (y) | Gender | Type of infection | Vintage on PD (months), (etiology) | PD method | Previous peritonitis |
|---|---|---|---|---|---|---|---|---|
| 1 | 1983, English7 | USA | 16 | M | Peritonitis | NA (FSGS) | CAPD | Multiple episodes (previous bacterial peritonitis 5m before) |
| 2 | 1986, English8 | USA | 15 | M | Peritonitis | 24 (NA) | PD | NA |
| 3 | 1991, English9 | USA | 16 | M | Peritonitis | 37 (SLE) | CAPD | NA |
| 4 | 1993, English10 | USA | 10 | M | Peritonitis | NA (prune-belly syndrome) | PD | NA |
| 5 | 2004, English11 | USA | 18 | F | Peritonitis | 72 (SLE) | PD | NA |
| 6 | 2005, English12 | USA | 14 | F | ESI | NA (SLE) | CCPD | 1 episode (previous bacterial peritonitis 6m before) |
| 7 | 2013, English13 | India | 9 | F | Peritonitis | 1 (chronic glomerulonephritis) | CAPD | No |
| 8 | 2014, English14 | Japan | 13 | F | Peritonitis | 5 (long-term nephrotoxic drugs due to unrelated BMT) | CAPD | No |
| 9 | 2015, Spanish15 | Spain | 4 | M | ESI | 4 (NA) | CAPD | No |
| 10 | 2015, English16 | Japan | 25m | F | ESI | 12 (complicated urinary tract malformations) | CAPD | No |
| 11 | 2015, English16 | Japan | 11m | F | ESI | 8 (bilateral hypo-dysplastic kidney) | CAPD | No |
| 12a | 2015, English16 | Japan | 26m | F | ESI | 23 (bilateral hypo-dysplastic kidney) | CAPD | 1 episode (previous ESI-M. chelonae 15m before) |
| 13 | 2015, English16 | Japan | 3m | M | ESI | 2 (angiotensin receptor blocker fetopathy) | CAPD | No |
| 14b | 2015, English16 | Japan | 5m | M | Peritonitis | 4 (angiotensin receptor blocker fetopathy) | CAPD | 1 episode (previous ESI-M. chelonae 2m before) |
| 15 | 2015, English16 | Japan | 23m | M | ESI | 22 (diffuse mesangial sclerosis) | CAPD | 1 episode (previous bacterial ESI 1m before) |
| 16 | 2015, English16 | Japan | 4m | F | ESI | 3 (right multicystic dysplastic and left hypo-dysplastic kidneys) | CAPD | 1 episode (previous bacterial ESI 1m before) |
| 17 | 2020, English17 | Qatar | 9 | M | Peritonitis | NA (bilateral multicystic dysplastic kidneys) | APD | recurrent ESIs episodes (previous bacterial ESI 8m before) |
| 18 | 2021, English18 | Singapore | 6 | F | Peritonitis/ESI | 36 (bilateral dysplastic kidneys) | APD | 2 ESIs episodes (previous bacterial ESI 1m before) |
| 19 | 2021, English19 | Japan | 4 | M | Peritonitis | NA (bilateral hypoplastic kidneys) | CAPD | 1 episode (previous bacterial peritonitis 2w before) |
| 20 | 2022, English20 | Canada | 9 | M | Peritonitis | 5 (RT as a result of allograft failure from T-cell–mediated rejection and BK virus nephropathy) | PD | 1 episode (previous bacterial peritonitis 1w before) |
| 21 | 2022, English21 | Japan | 32m | M | Peritonitis/ESI | 24 (bilateral hypodysplastic kidneys) | APD | 5 episodes (previous bacterial peritonitis 2m before) |
| 22 | 2024, English22 | USA | 9 | M | Peritonitis | 10 (reflux nephropathy) | APD | 1 episode (previous bacterial peritonitis 4m before) |
| 23 | 2024, English23 | Japan | 3 | M | Peritonitis/ESI | 17 (FSGS) | PD | No |
| 24 | 2024, English24 | Japan | 10 | M | ESI | 12 (juvenile nephronophthisis) | CCPD | No |
| 25 | 2025, English25 | Saudi Arabia | 8 | F | Peritonitis | NA (Cystic nephropathy/Obstructive uropathy) | PD | NA |
NTM, nontuberculous Mycobacteria; PD, peritoneal dialysis; NA, not available; CAPD, continuous ambulatory peritoneal dialysis; SLE, systemic lupus erythematosus; CCPD, continuous cycling peritoneal dialysis; BMT, bone marrow transplantation; ESI, exit site infection; APD, ambulatory peritoneal dialysis; RT, renal transplantation; FSGS, focal segmental glomerulosclerosis.
Diseases leading to ESRD were described in 23 of the 25 reviewed cases, in which was found that was secondary to congenital anomalies of the kidney and urinary tract (CAKUT) in 9 cases (39.1%), especially hypo- and or dysplastic kidney in 7 out of them. Twelve (52%) of 25 reported cases experienced peritonitis, 9 (36%) cases experienced ESI and the rest 3 (12%) cases suffered by both peritonitis/ESI.
Mean of vintage on PD in 19 cases with reported data was 16.89 months (range, 1 month–72 months), while in 11/19 (57.9%) vintage duration was equal or less than 12 months. Continuous ambulatory peritoneal dialysis (CAPD) was the commonest PD method used in 52% of cases. The clinical profile of PD-associated with NTM infections was non-specific, specifically, fever (52%), abdominal pain (44%), cloudy effluent (40%) were the most common manifestations. However, in 12 cases with PD-associated with NTM infections including ESI, either alone or combined with peritonitis, 91.7% of cases showed inflammatory skin lesions or were purulent.
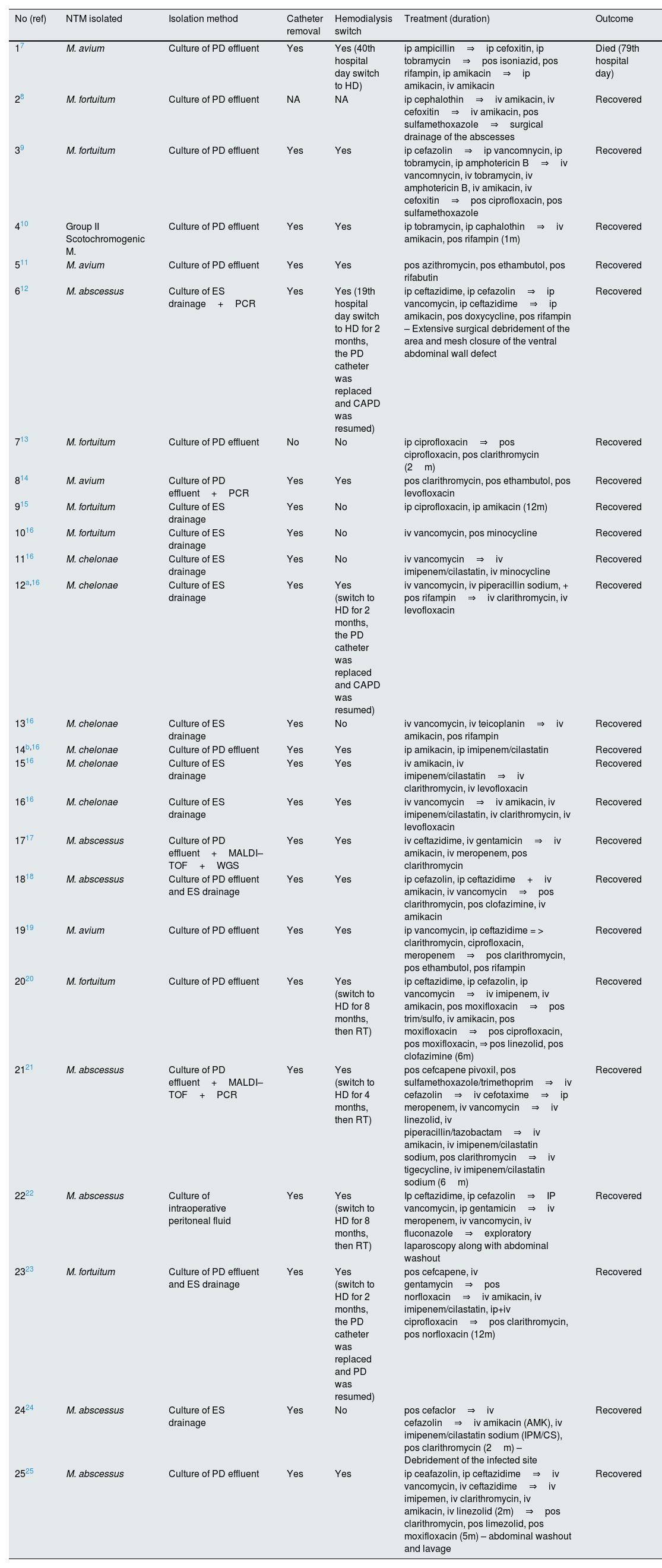
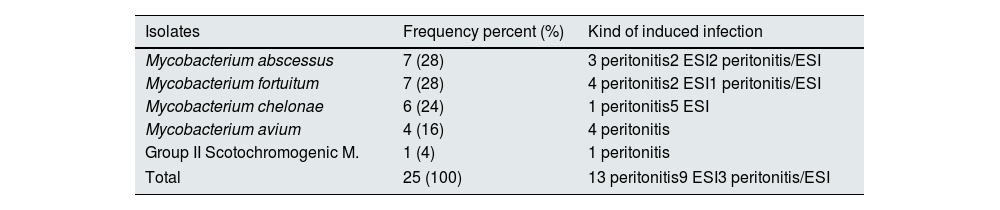
Microbiological findings and management of 25 cases of PD–related infections caused by NTM presented in Table 2. The most prevalent organisms responsible for PD-associated with NTM infections was M.abscessus and M. fortuitum (28%), each, followed by M.chelonae (24%) and M. avium (16%) (Table 3). M. chelonae PD-associated infections occurred in children with a mean age of 12 months (range, 3 months–26 months), constituting the NTM pathogen which affects the youngest age group among patients reviewed. Only cases with confirmed peritoneal effluent or exit site tissue culture were included in this review. The pathogens were further verified by molecular methods (PCR) in 3 of the 25 cases, 2 cases by MALDI–TOF analysis and 1 case by WGS method.
Microbiological findings and management of 24 cases of nontuberculous Mycobacteria peritoneal dialysis-related infections.
| No (ref) | NTM isolated | Isolation method | Catheter removal | Hemodialysis switch | Treatment (duration) | Outcome |
|---|---|---|---|---|---|---|
| 17 | M. avium | Culture of PD effluent | Yes | Yes (40th hospital day switch to HD) | ip ampicillin⇒ip cefoxitin, ip tobramycin⇒pos isoniazid, pos rifampin, ip amikacin⇒ip amikacin, iv amikacin | Died (79th hospital day) |
| 28 | M. fortuitum | Culture of PD effluent | NA | NA | ip cephalothin⇒iv amikacin, iv cefoxitin⇒iv amikacin, pos sulfamethoxazole⇒surgical drainage of the abscesses | Recovered |
| 39 | M. fortuitum | Culture of PD effluent | Yes | Yes | ip cefazolin⇒ip vancomnycin, ip tobramycin, ip amphotericin B⇒iv vancomnycin, iv tobramycin, iv amphotericin B, iv amikacin, iv cefoxitin⇒pos ciprofloxacin, pos sulfamethoxazole | Recovered |
| 410 | Group II Scotochromogenic M. | Culture of PD effluent | Yes | Yes | ip tobramycin, ip caphalothin⇒iv amikacin, pos rifampin (1m) | Recovered |
| 511 | M. avium | Culture of PD effluent | Yes | Yes | pos azithromycin, pos ethambutol, pos rifabutin | Recovered |
| 612 | M. abscessus | Culture of ES drainage+PCR | Yes | Yes (19th hospital day switch to HD for 2 months, the PD catheter was replaced and CAPD was resumed) | ip ceftazidime, ip cefazolin⇒ip vancomycin, ip ceftazidime⇒ip amikacin, pos doxycycline, pos rifampin – Extensive surgical debridement of the area and mesh closure of the ventral abdominal wall defect | Recovered |
| 713 | M. fortuitum | Culture of PD effluent | No | No | ip ciprofloxacin⇒pos ciprofloxacin, pos clarithromycin (2m) | Recovered |
| 814 | M. avium | Culture of PD effluent+PCR | Yes | Yes | pos clarithromycin, pos ethambutol, pos levofloxacin | Recovered |
| 915 | M. fortuitum | Culture of ES drainage | Yes | No | ip ciprofloxacin, ip amikacin (12m) | Recovered |
| 1016 | M. fortuitum | Culture of ES drainage | Yes | No | iv vancomycin, pos minocycline | Recovered |
| 1116 | M. chelonae | Culture of ES drainage | Yes | No | iv vancomycin⇒iv imipenem/cilastatin, iv minocycline | Recovered |
| 12a,16 | M. chelonae | Culture of ES drainage | Yes | Yes (switch to HD for 2 months, the PD catheter was replaced and CAPD was resumed) | iv vancomycin, iv piperacillin sodium, + pos rifampin⇒iv clarithromycin, iv levofloxacin | Recovered |
| 1316 | M. chelonae | Culture of ES drainage | Yes | No | iv vancomycin, iv teicoplanin⇒iv amikacin, pos rifampin | Recovered |
| 14b,16 | M. chelonae | Culture of PD effluent | Yes | Yes | ip amikacin, ip imipenem/cilastatin | Recovered |
| 1516 | M. chelonae | Culture of ES drainage | Yes | Yes | iv amikacin, iv imipenem/cilastatin⇒iv clarithromycin, iv levofloxacin | Recovered |
| 1616 | M. chelonae | Culture of ES drainage | Yes | Yes | iv vancomycin⇒iv amikacin, iv imipenem/cilastatin, iv clarithromycin, iv levofloxacin | Recovered |
| 1717 | M. abscessus | Culture of PD effluent+MALDI–TOF+WGS | Yes | Yes | iv ceftazidime, iv gentamicin⇒iv amikacin, iv meropenem, pos clarithromycin | Recovered |
| 1818 | M. abscessus | Culture of PD effluent and ES drainage | Yes | Yes | ip cefazolin, ip ceftazidime+iv amikacin, iv vancomycin⇒pos clarithromycin, pos clofazimine, iv amikacin | Recovered |
| 1919 | M. avium | Culture of PD effluent | Yes | Yes | ip vancomycin, ip ceftazidime = > clarithromycin, ciprofloxacin, meropenem⇒pos clarithromycin, pos ethambutol, pos rifampin | Recovered |
| 2020 | M. fortuitum | Culture of PD effluent | Yes | Yes (switch to HD for 8 months, then RT) | ip ceftazidime, ip cefazolin, ip vancomycin⇒iv imipenem, iv amikacin, pos moxifloxacin⇒pos trim/sulfo, iv amikacin, pos moxifloxacin⇒pos ciprofloxacin, pos moxifloxacin, ⇒ pos linezolid, pos clofazimine (6m) | Recovered |
| 2121 | M. abscessus | Culture of PD effluent+MALDI–TOF+PCR | Yes | Yes (switch to HD for 4 months, then RT) | pos cefcapene pivoxil, pos sulfamethoxazole/trimethoprim⇒iv cefazolin⇒iv cefotaxime⇒ip meropenem, iv vancomycin⇒iv linezolid, iv piperacillin/tazobactam⇒iv amikacin, iv imipenem/cilastatin sodium, pos clarithromycin⇒iv tigecycline, iv imipenem/cilastatin sodium (6m) | Recovered |
| 2222 | M. abscessus | Culture of intraoperative peritoneal fluid | Yes | Yes (switch to HD for 8 months, then RT) | Ip ceftazidime, ip cefazolin⇒IP vancomycin, ip gentamicin⇒iv meropenem, iv vancomycin, iv fluconazole⇒exploratory laparoscopy along with abdominal washout | Recovered |
| 2323 | M. fortuitum | Culture of PD effluent and ES drainage | Yes | Yes (switch to HD for 2 months, the PD catheter was replaced and PD was resumed) | pos cefcapene, iv gentamycin⇒pos norfloxacin⇒iv amikacin, iv imipenem/cilastatin, ip+iv ciprofloxacin⇒pos clarithromycin, pos norfloxacin (12m) | Recovered |
| 2424 | M. abscessus | Culture of ES drainage | Yes | No | pos cefaclor⇒iv cefazolin⇒iv amikacin (AMK), iv imipenem/cilastatin sodium (IPM/CS), pos clarithromycin (2m) – Debridement of the infected site | Recovered |
| 2525 | M. abscessus | Culture of PD effluent | Yes | Yes | ip ceafazolin, ip ceftazidime⇒iv vancomycin, iv ceftazidime⇒iv imipemen, iv clarithromycin, iv amikacin, iv linezolid (2m)⇒pos clarithromycin, pos limezolid, pos moxifloxacin (5m) – abdominal washout and lavage | Recovered |
NTM, nontuberculous Mycobacteria; PD, peritoneal dialysis; ip, intraperitoneal; pos, per os; iv, intravenous; NA, not available; CAPD, continuous ambulatory peritoneal dialysis; RT renal transplantation; HD, hemodialysis; ES, exit site; MALDI–TOF, matrix-assisted laser-desorption ionization–time of-flight mass spectrometry; WGS, whole-genome sequencing, m, months;
Data of isolates from 25 cases of peritoneal dialysis-related infections due to nontuberculous Mycobacteria and kind of induced infection.
| Isolates | Frequency percent (%) | Kind of induced infection |
|---|---|---|
| Mycobacterium abscessus | 7 (28) | 3 peritonitis2 ESI2 peritonitis/ESI |
| Mycobacterium fortuitum | 7 (28) | 4 peritonitis2 ESI1 peritonitis/ESI |
| Mycobacterium chelonae | 6 (24) | 1 peritonitis5 ESI |
| Mycobacterium avium | 4 (16) | 4 peritonitis |
| Group II Scotochromogenic M. | 1 (4) | 1 peritonitis |
| Total | 25 (100) | 13 peritonitis9 ESI3 peritonitis/ESI |
ESI, exit-site infection.
In 23 (95.8%) of 24 cases with reported data, the PD catheter was removed and patients were given a novel PD catheter (25%) continuing PD methodology or most commonly switched to hemodialysis (75%). In 3 cases, a transition from PD to hemodialysis was necessitated and this was subsequently followed by successful renal transplantation, highlighting a favorable clinical course despite the complexity of their prior management.
All patient initially received empirical antibacterial treatment, which was subsequently modified in the majority of cases to ensure adequate coverage against NTM, following mycobacterial identification and susceptibility testing. The treatment duration ranged from 1 month to 12 months with a mean and median of the treatment, in cases with reported data, being 5.6 and 6 months, respectively. The anti-NTM regimen was selected according to specific isolates and/or in vitro anti-mycobacterial susceptibility. The most commonly used agents for the treatment of PD-associated with NTM infections included macrolides, aminoglycosides, fluoroquinolones, tetracyclines, carbapenems, oxazolidinones, rifampin, ethambutol and rifabutin. The outcome was favorable in all cases, with the sole exception of one patient who unfortunately died due to the infection. Notably, this particular case represented the first ever published report of PD-associated with NTM infections more than four decades ago.7
DiscussionOur systematic review revealed 25 pediatric cases of PD-associated with NTM infections published to date, most of them reported as isolated case reports. M. abscessus and M. fortuitum were the predominant species in this cohort mirrors trends in both adult and pediatric populations.26,27 However, there appears to be a shift in recent years toward broader recognition of species such as M. chelonae and M. avium, partly due to improved identification techniques including MALDI–TOF and PCR.4 Diagnostic delays remain a major issue, with initial cultures often misidentified as diphtheroids or contaminated samples.1 Furthermore, the lack of clinical suspicion, especially in patients presenting with mild or atypical symptoms, such as localized exit-site inflammation without systemic signs, contributes to diagnostic inertia.
Differentiating PD-related infections caused by NTM from those due to conventional bacterial pathogens remains a significant clinical challenge but is essential for timely diagnosis and effective management. While both entities can present with non-specific symptoms such as fever, abdominal pain or cloudy effluent, a key distinguishing feature of NTM infections is their poor or absent response to empirical broad-spectrum antibiotics, which should raise early clinical suspicion in refractory cases.1,2 Diagnostic delays are common, as standard microbiological methods may fail to detect NTM or misidentify them as contaminants, underscoring the need for acid-fast staining and specialized culture techniques, or advanced modalities such as MALDI–TOF, PCR or WGS.4,27 Although epidemiological differences in age or PD vintage between NTM and common bacterial infections are not consistently pronounced, our review shows that NTM infections more frequently require PD catheter removal and a temporary shift to hemodialysis, suggesting a more complex clinical course.3,25,26 Taken together, these factors emphasize the importance of maintaining a high index of suspicion for NTM, particularly in cases unresponsive to standard treatment protocols, to ensure prompt diagnostic confirmation and implementation of appropriate therapy.
The reviewed pediatric cases revealed substantial variation in clinical presentation and therapeutic approaches. Peritonitis was the most frequent manifestation, although ESI were also common. In our study, the mean duration on PD prior to the onset of NTM infection was approximately 17 months, with a substantial proportion of cases occurring within the first year following the initiation of PD as renal replacement therapy. This suggests a potential role of early technique training, catheter care protocols and environmental exposure in disease pathogenesis.2,26 Fever, abdominal pain and cloudy effluent were common but not universal findings. Interestingly, most cases involving ESI were accompanied by skin lesions or purulent discharge, highlighting the importance of local signs in early suspicion of NTM.26
From a microbiological standpoint, culture positivity from peritoneal fluid or exit-site samples remains the cornerstone of diagnosis. However, newer techniques such as MALDI–TOF and PCR provide more rapid and accurate identification, especially in slow-growing or atypical isolates like mycobacteria.4 The use of WGS remains limited in routine clinical practice, but may become more accessible in tertiary centers in the near future and it will probably be the method of choice for the detection of these pathogens.
Treatment strategies varied considerably, both in terms of antibiotic selection and therapy duration. According to the ISPD guidelines, at least two antimycobacterial agents with in vitro activity against the isolate should be used, ideally for a minimum of four months.2 However, in practice as was found in our study, therapy duration ranged from a few weeks to several months and a wide variety of drug combinations, which were often empirical due to limited susceptibility data, were administered. In general, macrolides, fluoroquinolones, amikacin and linezolid were the most frequently used agents in literature, either alone or in combination, which are in accordance with our findings.25,26
Catheter removal was performed in the majority of cases, often after failure of initial empirical therapy. Early removal may be advisable, especially in tunnel or ESI, to prevent progression to peritonitis or systemic dissemination. However, the optimal timing of catheter removal and subsequent reinsertion remains poorly defined in the pediatric setting and should be individualized based on clinical response and microbial eradication. While catheter salvage is possible in select cases of localized ESI, relapsing infections and persistent inflammation frequently necessitate catheter replacement or a temporary switch to hemodialysis.1,4,27
The long-term prognosis of pediatric patients with NTM-related PD infections is generally favorable when appropriately treated, although some cases are associated with PD method failure and transition to hemodialysis. There are no clear recommendations on the optimal timing for reinitiating PD after catheter removal due to NTM infection and decisions are often individualized. Given the chronic nature of the underlying kidney disease in most of these children, preserving peritoneal membrane function and minimizing disruptions in dialysis modality are critical considerations.1,26
This study is limited by the small sample size, reflecting the rarity of PD-related infections due to NTM. The retrospective nature of case reports and the heterogeneity of data present significant challenges. This includes the infrequent reporting of specific peritoneal dialysis solution types in the included case reports, which prevented a comprehensive analysis of their potential influence on infection risk. Furthermore, incomplete reporting on treatment regimens, duration, and outcomes in some cases limits our ability to draw firm conclusions about optimal management strategies. Another emerging issue is the potential underreporting of cases. Given the difficulty in diagnosis and frequent misidentification, the true incidence of NTM-related PD infections may be underestimated, especially in resource-limited settings. Moreover, the inclusion of cases only with confirmed positive cultures may lead to selection bias in existing literature. However, despite these limitations, our study contributes valuable insights into an underreported condition and supports the need for heightened clinical suspicion and tailored microbiological evaluation in pediatric PD patients.
Notwithstanding the slowly growing number of case reports, there remains a lack of high-quality evidence and consensus on optimal management. The heterogeneity in treatment approaches highlights the need for standardized treatment protocols based on susceptibility data and pediatric pharmacokinetics. Future research should aim to establish prospective registries and multicenter collaborations to gather more robust data on incidence, outcomes and antimicrobial susceptibility. Until then, clinical suspicion, early diagnosis, aggressive therapy with appropriate agents and timely catheter management remain the cornerstones of successful outcomes.
- 1.
PD-related infections due to NTM in children and adolescents are rare but clinically significant, requiring heightened diagnostic suspicion and vigilance.
- 2.
The predominant pathogens were M. abscessus, M. fortuitum and M.chelonae, often associated with rapid disease progression.
- 3.
Peritonitis and ESIs due to NTM presented with non-specific symptoms.
- 4.
Catheter removal was needed in the majority of cases with most of patients switched to hemodialysis, while a few resumed PD.
- 5.
Treatment required prolonged, species-specific antibiotic regimens, with outcomes generally be favorable.
The authors declare that they have no potential conflict of interest related to the contents of this article.