Acute renal infarction (ARI) is an uncommon disease, whose real incidence is probably higher than expected. It is associated with poor prognosis in a high percentage of cases.
ObjectivesTo describe the main clinical, biochemical and radiologic features and to determine which factors are associated with poor prognosis (death or permanent renal injury).
Materials and methodsThe following is a retrospective, observational, single-center study. All patients diagnosed with ARI by contrast-enhanced computed tomography (CT) over an 18-year period were included. Patients were classified according to the cardiac or non-cardiac origin of their disease. Clinical, biochemical and radiologic features were analysed, and multiple logistic regression model was used to determine factors associated with poor prognosis.
ResultsA total of 62 patients were included, 30 of which had a cardiac origin. Other 32 patients with non-cardiac ARI were younger, had less comorbidity, and were less frequently treated with oral anticoagulants. CT scans estimated mean injury extension at 35%, with no differences observed between groups. A total of 38% of patients had an unfavourable outcome, and the main determinants were: Initial renal function (OR=0.949; CI 95% 0.918–0.980; p=0.002), and previous treatment with oral anticoagulants (OR=0.135; CI 95% 0.032–0.565; p=0.006).
ConclusionsARI is a rare clinical condition with non-specific symptoms, and it is not associated with cardiological disease or arrhythmias in more than half of cases. A substantial proportion of patients have unfavourable outcomes, and the initial renal function is one of the main prognostic factors.
El infarto renal agudo (INRA) es una patología de diagnóstico infrecuente, cuya incidencia real es probablemente superior a la detectada, y que asocia una evolución desfavorable en un alto porcentaje de casos.
ObjetivosDescribir las principales características clínicas, bioquímicas y radiológicas, y determinar qué factores se asocian a una peor evolución (muerte o deterioro permanente de la función renal).
Material y métodosEstudio retrospectivo y observacional, que incluyó a todos los pacientes diagnosticados de INRA mediante TAC con contraste en un único hospital durante 18 años. Los pacientes fueron clasificados según el origen cardiogénico o no cardiogénico del INRA. Se analizaron las principales características clínicas, bioquímicas y radiológicas, y, mediante un modelo de regresión logística multivariante, se determinaron los factores asociados a una peor evolución.
ResultadosSe incluyeron 62 casos, de los que 30 fueron de origen cardiogénico. Los 32 pacientes con INRA no cardiogénico eran más jóvenes, con menos comorbilidad y menor frecuencia de tratamiento previo con anticoagulación. La extensión media de daño isquémico por radiología fue del 35%, sin observarse diferencias entre los subgrupos etiológicos. El 38% de los pacientes tuvo una evolución desfavorable, y los principales determinantes fueron: la función renal al diagnóstico (eGFR) (OR=0,949; IC 95%: 0,918–0,980; p=0,002) y la anticoagulación oral antes del episodio agudo (OR=0,135; IC 95%: 0,032–0,565; p=0,006).
ConclusionesEl INRA es una patología infrecuente, con manifestaciones clínicas poco específicas y, en más de la mitad de los casos, no asociada a enfermedad cardiaca o arritmias. Una alta proporción de pacientes evoluciona desfavorablemente. La función renal al diagnóstico es uno de los principales factores pronósticos.
Acute renal infarction (ARI) refers to ischaemic damage of the renal parenchyma caused by the sudden interruption of blood flow. It is a rare cause of acute kidney failure, with an incidence rate in published series ranging from 0.007% to 1.4%.1,2 However, its incidence is probably higher, due to its difficult diagnosis.
It is commonly manifested by the sudden onset of diffuse abdominal or lumbar pain together with neurovegetative symptoms that cannot be well controlled with analgesics; occasionally fever is present. This set of non-specific symptoms is some times interpreted as other common conditions such as urolithiasis, pyelonephritis or gastrointestinal diseases.3
The two major causes of ARI are renal embolism, often originated at the heart and triggered by atrial fibrillation, and renal artery thrombosis, caused by traumatic lesions or lesions originated during diagnostic and therapeutic procedures. Other common causes include renal artery dissection or fibromuscular dysplasia and spontaneous thrombosis associated with hypercoagulability. Nevertheless, the cause of ARI is impossible to determine in 29–59% of cases.4–8 Early treatment with anticoagulants or fibrinolytics may reverse the ischaemia and improve prognosis.9,10 Unfortunately, diagnosis is often delayed, which is why ARI should always be included in the differential diagnosis of patients presenting with the aforementioned symptoms.
The present study includes of 62 patients with ARI in native kidneys (excluding transplanted kidneys) diagnosed at Hospital Ramón y Cajal over an 18-year period. The study objectives were to describe the main clinical, biochemical and radiological features of this disease, to analyse clinical differences based on aetiology and to determine which factors are associated with poor prognosis (death or permanent renal function impairment).
Material and methodsARI patients diagnosed by radiological methods from 1996 to 2014 were selected. Kidney transplant patients were excluded.
Baseline characteristics were obtained from the medical records, including demographic data, the Charlson Comorbidity Index, cardiovascular risk factors (history of hypertension, diabetes mellitus, dyslipidaemia, smoking, ischaemic heart disease, arrhythmias, thrombophilia and previous embolisms), history of chronic kidney disease and previous treatment with antiplatelet agents or oral anticoagulation was also recorded.
ARI was diagnosed by axial or spiral computed tomography (CT scan) depending on the study period and with the administration of an intravenous contrast.
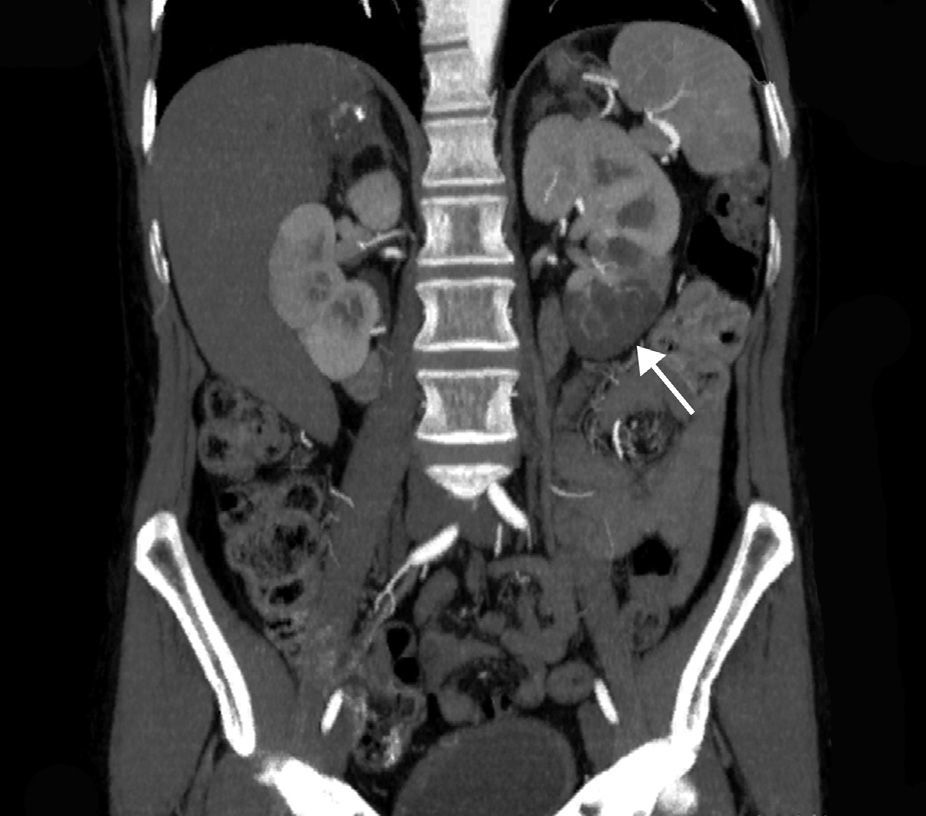
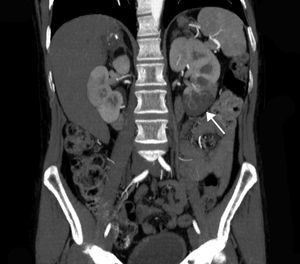
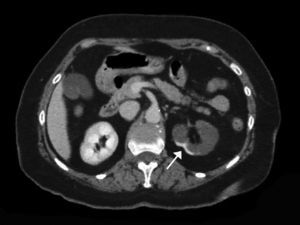
The extent of ischaemic injury was estimated in each patient by dividing the kidney into six segments, such that ARI of the renal poles, with no involvement of the rest of the kidney or the contralateral kidney, was considered to have 1/6 degree of damage (17%) (Figs. 1 and 2). Thus, the estimated injury extent for each patient could theoretically range from 1/6 (17%) to 6/6 (100%).
Baseline lab work included: haematological parameters (haemoglobin, total leucocyte count, neutrophil and platelet counts); and biochemical parameters with serum creatinine, lactate dehydrogenase (LDH) and C-reactive protein (CRP). Renal function was estimated using the glomerular filtration rate (eGFR) by applying the 4-variable MDRD formula.11 Impaired renal function was defined as an eGFR of <60ml/min/1.73m2 at diagnosis or during the first week of progression in all patients with no previous history of kidney disease. Conventional laboratory methods were applied, although they differed depending on the year in which the study events occurred.
Patients were assigned to 2 subgroups according to the cardiac or non-cardiac origin of their ARI, and the characteristics of these subgroups were compared.
The outcome data was censored 6 months after the initial onset of ARI. Unfavourable outcome was deemed to be death from any cause or the non-recovery of partial or total renal function after 6 months of the event. Partial recovery was defined as renal impairment (eGFR<60ml/min/1.73m2), or an eGFR value lower than the figure obtained before the onset of ARI in patients with a known history of renal impairment.
Study design and statistical analysisThis is a retrospective, observational study that describes the clinical and biochemical characteristics of patients diagnosed with ARI.
Student's t-test or the Mann–Whitney non-parametric test was used to compare 2 continuous independent variables for unpaired samples, depending on the distribution characteristics of the variables. The chi-square test with continuity correction was used to compare discrete variables.
A multivariate logistic regression model was used to establish the clinical parameters associated to an unfavourable progression following acute renal infarction, and the following independent variables were considered: age, gender, comorbidity index, days since the onset of symptoms to treatment initiation, aetiological origin (cardiac or non-cardiac), glomerular filtration rate at diagnosis, total leucocyte count, LDH, antiplatelet agents and oral anticoagulation prior to the acute episode.
To prevent collinearity, the model excluded neutrophil count and the extent of kidney damage by radiology (see Results section). To prevent overfitting, variables were forced into the multivariate model with a significance of at least p<0.01. The variables in the multivariate model were chosen automatically by conditional progressive inclusion (forward).
Data were presented as mean and standard deviation (±SD) or as median and interquartile ranges or maximum and minimum values. p<0.05 was considered to be statistically significant.
IBM SPSS version 21 software was used to conduct the statistical analysis.
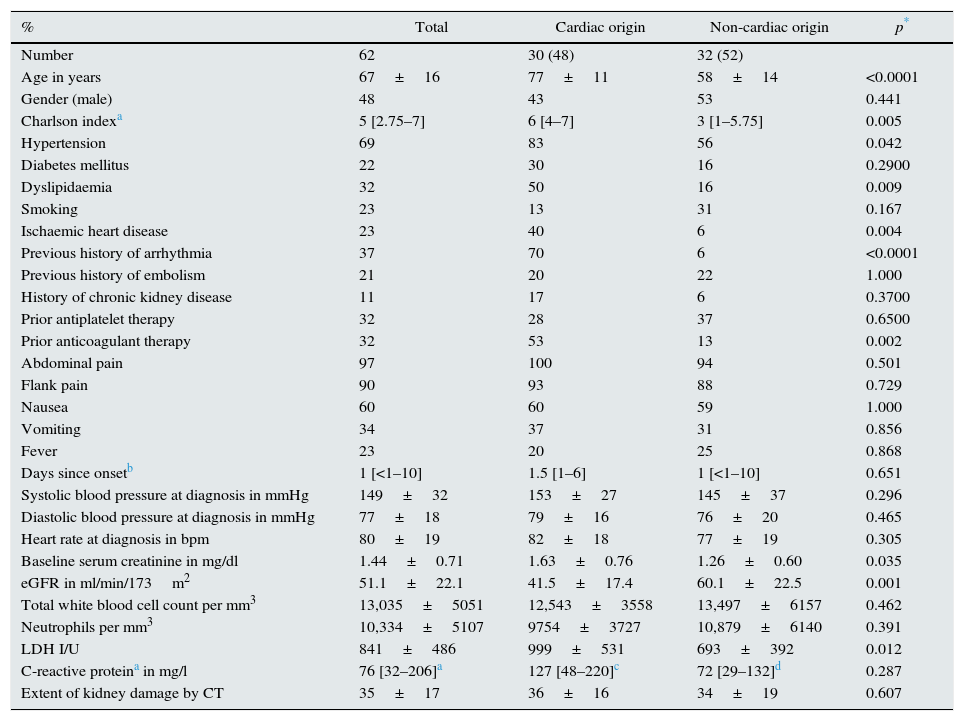
ResultsDemographic and clinical characteristics of the ARI patients62 patients diagnosed with ARI in native kidneys were identified in the study period. The demographic and clinical characteristics are shown in Table 1.
Demographic and clinical characteristics of all patients and by aetiological subgroup.
| % | Total | Cardiac origin | Non-cardiac origin | p* |
|---|---|---|---|---|
| Number | 62 | 30 (48) | 32 (52) | |
| Age in years | 67±16 | 77±11 | 58±14 | <0.0001 |
| Gender (male) | 48 | 43 | 53 | 0.441 |
| Charlson indexa | 5 [2.75–7] | 6 [4–7] | 3 [1–5.75] | 0.005 |
| Hypertension | 69 | 83 | 56 | 0.042 |
| Diabetes mellitus | 22 | 30 | 16 | 0.2900 |
| Dyslipidaemia | 32 | 50 | 16 | 0.009 |
| Smoking | 23 | 13 | 31 | 0.167 |
| Ischaemic heart disease | 23 | 40 | 6 | 0.004 |
| Previous history of arrhythmia | 37 | 70 | 6 | <0.0001 |
| Previous history of embolism | 21 | 20 | 22 | 1.000 |
| History of chronic kidney disease | 11 | 17 | 6 | 0.3700 |
| Prior antiplatelet therapy | 32 | 28 | 37 | 0.6500 |
| Prior anticoagulant therapy | 32 | 53 | 13 | 0.002 |
| Abdominal pain | 97 | 100 | 94 | 0.501 |
| Flank pain | 90 | 93 | 88 | 0.729 |
| Nausea | 60 | 60 | 59 | 1.000 |
| Vomiting | 34 | 37 | 31 | 0.856 |
| Fever | 23 | 20 | 25 | 0.868 |
| Days since onsetb | 1 [<1–10] | 1.5 [1–6] | 1 [<1–10] | 0.651 |
| Systolic blood pressure at diagnosis in mmHg | 149±32 | 153±27 | 145±37 | 0.296 |
| Diastolic blood pressure at diagnosis in mmHg | 77±18 | 79±16 | 76±20 | 0.465 |
| Heart rate at diagnosis in bpm | 80±19 | 82±18 | 77±19 | 0.305 |
| Baseline serum creatinine in mg/dl | 1.44±0.71 | 1.63±0.76 | 1.26±0.60 | 0.035 |
| eGFR in ml/min/173m2 | 51.1±22.1 | 41.5±17.4 | 60.1±22.5 | 0.001 |
| Total white blood cell count per mm3 | 13,035±5051 | 12,543±3558 | 13,497±6157 | 0.462 |
| Neutrophils per mm3 | 10,334±5107 | 9754±3727 | 10,879±6140 | 0.391 |
| LDH I/U | 841±486 | 999±531 | 693±392 | 0.012 |
| C-reactive proteina in mg/l | 76 [32–206]a | 127 [48–220]c | 72 [29–132]d | 0.287 |
| Extent of kidney damage by CT | 35±17 | 36±16 | 34±19 | 0.607 |
The number of ARI diagnoses increased with each passing year of the study period. By analysing ARI prevalence in 4-year periods, the vast majority (48 cases) were diagnosed in the last 8 years.
Embolic ARI of cardiac origin was diagnosed in 30 patients (48%). Atrial fibrillation was the only associated arrhythmia, and ARI was the clinical manifestation of this arrhythmia in 48% of cases. The triggering factor leading to embolism formation was probably poor anticoagulation control. There were no cases of endocarditis.
The associated conditions of the 32 cases (52%) of ARI of non-cardiac origin were: damage or lesion of the renal arteries caused by aortic and renal dissection (6 cases); post abdominal surgery (4 cases); polyarteritis nodosa (1 case); renal artery stenosis/thrombosis (3 cases); thrombophilia (1 case of polycythaemia and 7 cases of hereditary coagulation disorders); septic shock (1 case), neoplasms (5 cases) and unknown origin (4 cases).
Patients with ARI of non-cardiac origin were younger, with fewer comorbidities and fewer cardiovascular risk factors than patients with ARI of cardiac origin (Table 1). 53% of patients with ARI of cardiac origin were already being treated with oral anticoagulants versus just 13% of patients with ARI of non-cardiac origin. The only anticoagulant administered was acenocoumarol. No differences in treatment rates with antiplatelet agents were observed.
95% of patients with ARI of cardiac origin who were taking oral anticoagulation had an INR below the therapeutic range at diagnosis. Just one patient with ARI of cardiac origin had an INR within therapeutic range at diagnosis, although symptoms had manifested 72h previously.
87% of patients were diagnosed in the Emergency Department and the remainder in an inpatient setting (Internal Medicine, Surgery). No significant differences in diagnostic delay between the inpatient setting and the emergy department were observed.
The most common symptoms were abdominal pain, flank pain and nausea (Table 1). Vomiting and fever were less common. No differences in general symptoms were observed between patients with ARI of cardiac and non-cardiac origin (Table 1). There was also no difference between the subgroups in terms of diagnostic delay, which ranged from fewer than 2h to 10 days, with a median of 1–1.5 days.
Impaired kidney function at diagnosis was observed in 35 patients (65%) with no previous history of kidney disease. Kidney function (eGFR) at the start of the episode was significantly lower, and serum LDH concentrations higher, in patients with ARI of cardiac origin (Table 1). However, the leucocyte, neutrophil and C-reactive protein counts did not differ between the subgroups.
Estimated mean extent of ischaemic damage measured by CT scan was 35% (2/6 of the total renal parenchyma), with peripheral wedge-shaped hypoperfusion present in 70% of cases, global involvement in 21% of cases and multifocal involvement in 9% of cases. All patients had two native kidneys and there were no cases of previously reported renal atrophy. No differences were found between the subgroups in terms of predominant involvement of the left or right kidney (Table 1).
There was an inverse and linear correlation between this radiological assessment of ischaemic damage and the eGFR (R=−0.39; p=0.001).
Following ARI diagnosis, anticoagulation with intravenous heparin sodium was started (to try to maintain activated thromboplastin time at 1.5–2 times normal), before introducing oral anticoagulation (acenocoumarol to achieve INR between 2 and 2.5). Only 4 patients underwent local fibrinolysis with urokinase infusion (starting dose of 200,000IU and subsequent perfusion of 100,000IU/h for 6h). Use of fibrinolysis was conditioned by the experience of the attending medical team, given the associated risks, and particularly as ARI had occurred in less than 24h. Three out of the 4 patients, were successfully treated, with complete recovery of renal function. The fourth patient experienced upper gastrointestinal bleeding which prompted the suspension of the treatment. No patient underwent surgical revascularisation.
Determining factors for an unfavourable outcomeThe outcomes of 24 patients (39%) with ARI were unfavourable: 8 deaths, 5 end up with advanced renal failure and 14 cases had partially recovered renal function at 6 months (3 patients with kidney failure died).
Extrarenal embolisms (ischaemic stroke, mesenteric ischaemia, embolisms in the lower limbs), developed in 9 patients (15%) no differences were found between the subgroups.
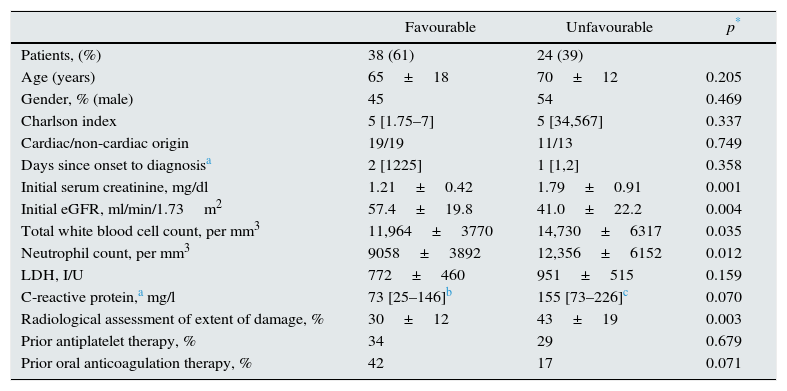
Table 2 shows the differences between patients with favourable and unfavourable combined clinical course. No demographic differences were found in the comorbidity index or in days since onset to diagnosis. Patients with poor clinical course had poorer initial renal function, significantly higher leucocyte and neutrophil blood counts and higher LDH and C-reactive protein concentrations than patients with good clinical course, although the differences in these 2 parameters were not statistically significant.
Clinical, biochemical and radiological characteristics of patients with favourable and unfavourable combined clinical course (death, or partial or total renal function impairment at 6 months post-ARI).
| Favourable | Unfavourable | p* | |
|---|---|---|---|
| Patients, (%) | 38 (61) | 24 (39) | |
| Age (years) | 65±18 | 70±12 | 0.205 |
| Gender, % (male) | 45 | 54 | 0.469 |
| Charlson index | 5 [1.75–7] | 5 [34,567] | 0.337 |
| Cardiac/non-cardiac origin | 19/19 | 11/13 | 0.749 |
| Days since onset to diagnosisa | 2 [1225] | 1 [1,2] | 0.358 |
| Initial serum creatinine, mg/dl | 1.21±0.42 | 1.79±0.91 | 0.001 |
| Initial eGFR, ml/min/1.73m2 | 57.4±19.8 | 41.0±22.2 | 0.004 |
| Total white blood cell count, per mm3 | 11,964±3770 | 14,730±6317 | 0.035 |
| Neutrophil count, per mm3 | 9058±3892 | 12,356±6152 | 0.012 |
| LDH, I/U | 772±460 | 951±515 | 0.159 |
| C-reactive protein,a mg/l | 73 [25–146]b | 155 [73–226]c | 0.070 |
| Radiological assessment of extent of damage, % | 30±12 | 43±19 | 0.003 |
| Prior antiplatelet therapy, % | 34 | 29 | 0.679 |
| Prior oral anticoagulation therapy, % | 42 | 17 | 0.071 |
The extent of ischaemic damage assessed by CT was significantly higher in patients with a worse clinical course.
Also of note was the greater use of prior oral anticoagulation in ARI patients with a good clinical course
Table 3 shows the degree of association of each of the variables assessed with poor combined outcome. The table also details the variables that went into the improved poor combined outcome predictive multivariate model.
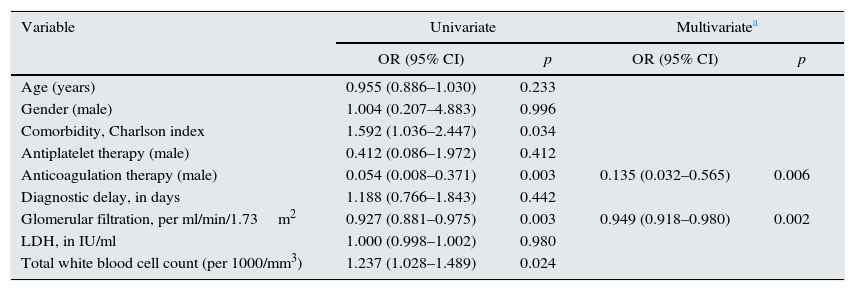
Univariate and multivariate logistic regression model of unfavourable combined outcomes (death or permanent renal function impairment) associated with acute renal infarction.
| Variable | Univariate | Multivariatea | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age (years) | 0.955 (0.886–1.030) | 0.233 | ||
| Gender (male) | 1.004 (0.207–4.883) | 0.996 | ||
| Comorbidity, Charlson index | 1.592 (1.036–2.447) | 0.034 | ||
| Antiplatelet therapy (male) | 0.412 (0.086–1.972) | 0.412 | ||
| Anticoagulation therapy (male) | 0.054 (0.008–0.371) | 0.003 | 0.135 (0.032–0.565) | 0.006 |
| Diagnostic delay, in days | 1.188 (0.766–1.843) | 0.442 | ||
| Glomerular filtration, per ml/min/1.73m2 | 0.927 (0.881–0.975) | 0.003 | 0.949 (0.918–0.980) | 0.002 |
| LDH, in IU/ml | 1.000 (0.998–1.002) | 0.980 | ||
| Total white blood cell count (per 1000/mm3) | 1.237 (1.028–1.489) | 0.024 | ||
The separate analysis of the cause of death within 6 months (8 cases) revealed the comorbidity index to be the only variable significantly associated with mortality (OR=1.60; 95% CI: 1.07–2.41; p=0.023).
The causes of persistent renal function impairment after excluding mortality at 6 months were the initial glomerular filtration (OR=0.804; 95% CI: 0.695–0.930; p=0.003) and the prior prescription of anticoagulants (OR=0.037; 95% CI: 0.002–0.803; p=0.036).
DiscussionThe results of this study show that ARI is an infrequent diagnosis, with non specific clinical manifestations and it is often associated to comorbid conditions (heart disease, arrhythmia, etc.), which eventually help the physicians to suspect the diagnosis. However, it is important to point out that more than half of the patients studied, particularly younger patients, had no associated cardiac conditions.
Approximately, 40% of patients had an unfavourable outcome in terms of survival or permanent renal impairment, and the main factors that determine poor outcome were the renal function at time of diagnosis and the lack of oral anticoagulant therapy prior to the onset of ARI.
This study compiled one of the largest series of ARI published cases at a single hospital site. Given that the population covered by the Hospital Ramón y Cajal is approximately 600,000, the prevalence of ARI can be estimated to be about 5.7 cases per million population/year. The actual frequency of ARI is probably higher taking into account the increase proportion of ageing population and the high prevalence of cardiovascular disease treated at the hospital, as well as potential unrecognised cases due to lack of ARI symptoms.2 It is impossible to determine whether the increasing prevalence of ARI throughout a given study period should be attributed to greater medical awareness or to an actual increase in ARI cases.
In our series, cardioembolic disease associated with atrial fibrillation was the most common cause of ARI. These findings differ from other reports by other, who found the most common aetiology is thrombosis caused by damage to the renal artery.4,5
Even after exhaustive diagnostic studies, the cause of ARI could not be determined in 4 cases.12,13 This diagnosis requires a high degree of suspicion in patients presenting with abdominal pain and particularly flank pain, and especially for patients with cardiovascular risk factors or at risk of suffering a thrombotic event.6,14
Renal function impairment in the context of ARI is largely due to renal hypoperfusion, although the associated symptoms (vomiting, reduced oral intake), or the use of intravenous contrast agents may be contributing factors. Determination of LDH has been shown to be a key diagnostic factor, as it increases in the first 24h and may remain high for up to 2 weeks.6,9,15
A urinalysis may also be useful in confirming a diagnosis. It has been reported that a bilateral obstruction of the renal arteries yields to a urine with sodium, creatinine and urea concentrations similar to plasma concentrations.16 Conversely, urine LDH levels may be higher than in plasma in cases of renal ischaemia.15 Urinalysis was not available in many patients in this study, nor was it possible to determine the proportion of patients with oliguria or haematuria. For this reason, urine was not included as a variable y the present study.
Diagnostic imaging is required to confirm an ARI diagnosis. There is no consensus in the literature as to the imaging technique of choice for a correct diagnosis, though most series used abdominal contrast-enhanced CT scan.4,6,7,9,17,18
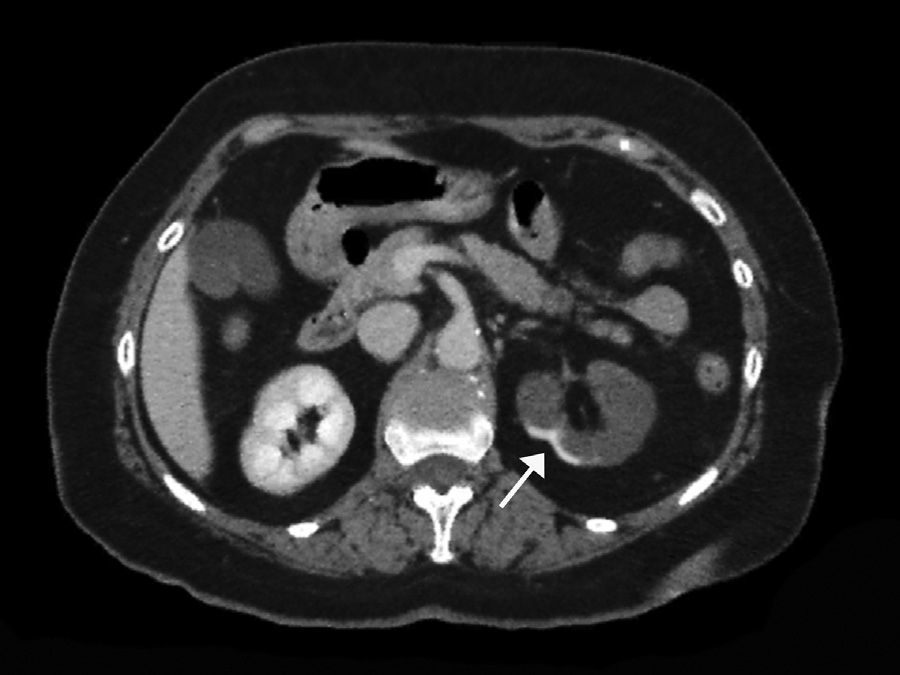
A contrast-enhanced CT scan reveals perfusion defects, sometimes accompanied by cortical rim sign, which correspond to the blood flow through collateral arteries19 (Fig. 2). If, intravenous contrast is not used, ARI may not be recognised.20
An angiography, on the other hand, is a diagnostic confirmation test, which also provides the location and extent of arterial occlusion in more detail. But the associated risks are also greater, including nephrotoxicity caused by greater exposure to the radiocontrast agent, atheroembolism or artery ruptures.13 Alternative techniques include radioisotope renography or renal Doppler ultrasound, although the latter, despite being affordable and safe, has not been proven to be sufficiently sensitive.9 Contrast-enhanced ultrasound seems to produce better results, but broader experience is required to validate its effectiveness as a diagnostic test.21
In this study, all cases were diagnosed with contrast-enhanced CT scan. The extent of ischaemic damage was similar in the cardiac and non-cardiac ARI subgroups, with findings similar to those reported from other series.20
Possible treatments include systemic anticoagulation, fibrinolysis and even open surgery in extremely rare cases. Fibrinolysis seems to be a good alternative, although time since onset and patient comorbidity should be taking into consideration before indicating fibrinolysis.22,23
Systemic anticoagulation is the standard treatment, especially for ARI of cardiac origin. In our series, just 4 patients underwent local fibrinolysis with urokinase infusion. In these cases, ARI diagnosis was made in an inpatient setting due to other causes, with just a few hours of delay in the diagnosis.
A noteworthy finding was the improved outcome of patients who were receiving anticoagulation therapy prior to ARI, despite the fact that prothrombin times were not always within optimal anticoagulation range. Hypotheses to justify this finding include: stronger diagnostic suspicion of ARI; smaller thrombi; and easiness and speed of achieve effective anticoagulation.
Thirty percent of patients diagnosed with ARI in our series remained with renal function impairment (CKD) 6 months after onset, which is similar to the findings in other studies.9,24 It has been reported that elevated creatinine levels at the onset predicts CKD.24 Patients frequently develop hypertension that is refractory to treatment.25
Overall mortality in this study was 13%, probably ARI was not the trigger of death but a consequence of the severe disease that the patient had (advanced cancer, sepsis or severe aortic dissection).5 The presence of extrarenal embolisms resulted in increased morbidity/mortality and length of hospital stay. Other studies have reported patient death after hospital discharge caused by ischaemic injury to other vital organs,18 which highlights the importance of correctly investigating embolic factors or the underlying cause of ARI in order to reduce associated mortality rates.5
This study has certain limitations. It is a retrospective study at a single hospital site, and it was not possible to collect data on all the variables from all the patients. Furthermore, diagnosis and follow-up was carried out by numerous medical teams, which means that patient management was neither uniform nor standardised by a protocol. The estimated extent of ischaemic damage by CT scan was not conducted using highest-precision methods (approximate estimation), although the significant correlation with baseline renal function is noteworthy.
In conclusion, although ARI is not a commonly diagnosed pathology, it should always be considered in the event of manifestation of certain symptoms or biochemical abnormalities, including in patients without a clinical history of heart disease or arrhythmia. Partial or total permanent kidney injury is a common complication, and early treatment could help to reduce the extent and severity of ischaemic renal damage.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Caravaca-Fontán F, Pampa Saico A, Elías Triviño S, Galeano Álvarez C, Gomis Couto A, Pecharromán de las Heras I, et al. Infarto renal agudo: características clínicas y factores pronósticos. Nefrología. 2016;36:141–148.