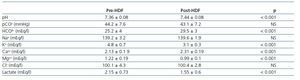
Introduction: The traditional evaluation of acid-base status relies on the Henderson-Hasselbach equation. In 1983, an alternative approach, based on physical and chemical principles was proposed by P. Stewart. In this approach, plasma pH is determined by 3 independent variables: pCO2, Strong Ion Difference (SIDm), which is the difference between the strong cations (Na+, K+, Ca++, Mg++) and the strong anions (Cl–, lactate) and total plasma concentration of nonvolatile weak acids (ATot), mainly inorganic phosphate and albumin. Bicarbonate is considered a dependent variable. The aim of this study was to evaluate the acid-base status using both perspectives, physical chemical and traditional approach. Material and methods: we studied 35 patients (24 male; 11 female) on hemodiafiltration, mean age was 67.2 ± 15.7, 8 ± 19.2 kg. We analyzed plasma chemistry including pH, pCO2, HCO3–, base excess and Na+, K+, Cl–, Ca++, Mg++, lactate and SIDm. The SID estimated (SIDe) was calculated by Figge’s formula (1,000 x 2.46–11 x pCO2/[10 – pH] + Album g/dl x [0.123 x pH –0.631] + P in mmol/l0 x [0.309 x pH –0.469]) and Gap of the SID as the difference SIDm-SIDe. Results: pH preHD was 7.36 ± 0.08 and pH post-HD 7.44 ± 0.08 (p <0.001). There was no significant differences between pCO2 pre- and post-HD. HCO3– and base excess increased during the session (p <0.001). SIDm decreased from 46.2 ± 2.9 pre-HD to 45 ± 2.3 mEq/l post-HD (p <0.05). On the opposite, SIDe increased from 38.5 ± 3.8 to 429 ± 3.1 mEq/l (p <0.001). The Gap Anion
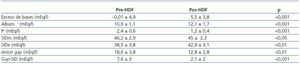
descended from 18.6 ± 3.8 pre-HD to 12.8 ± 2.8 mEq/l post-HD (p <0.001) and the Gap of the SID 7.6 ± 3 to 2.1 ± 2 (p <0.001). Anion Gap correlated with the Gap-SID so much pre-HDF as pos-HDF. ¿ Base excess correlated only with ¿ of the Gap SID. Conclusion: Stewart-Fencl’s approach does not improve characterization of acid-base status in patients on chronic HDF. In presence of normocloremia the SIDm does not reflect the alkalinizing process of the session of hemodialysis. According this approach, hemodialysis therapy can be viewed as a withdrawal of inorganic anions, especially the sulphate. These anions are replaced by OH– and secondarily for HCO3–. The approach only improves the evaluation of unmeasured anions by the Gap of the SID, without the effect of albumin and phosphate.
Introducción: la evaluación del equilibrio ácido-base se basa en la ecuación de Henderson-Hasselbach. En 1983, P. Stewart desarrolló un análisis cuantitativo del equilibrio ácido-base en el que muestra un sistema con unas variables independientes entre las que se incluyen pCO2, diferencia iónica fuerte medida (SIDm), es decir, la diferencia entre la suma de cationes fuertes (Na+, K+, Ca++, Mg++) y la suma de aniones fuertes (Cl–, lactato) y la concentración total de todos los aniones débiles no volátiles (ATot), cuyos principales representantes son el fósforo inorgánico (P–) y la albúmina (Albúm.–). El objetivo de este estudio es evaluar desde ambas perspectivas el equilibrio ácido-base en pacientes en hemodiafiltración (HDF) crónica. Material y métodos: se estudian 35 pacientes (24 hombres y 11 mujeres, con una edad media de 67,2 ± 15,7 años y con un peso seco de 72,8 ± 19,2 kg. La duración media de la hemodiálisis (HD) fue de 253,6 ± 40,5 minutos. Se analizan los parámetros gasométricos (pH, pCO2, HCO3–y exceso de bases) y Na+, K+, Cl–, Ca++, Mg++ y lactato. Se calcularon la SIDm, la SIDe mediante la fórmula de Figge (1.000 x 2,46–11 x pCO2 /[10 – pH] + Albúm. g/dl x [0,123 x pH –0,631] + P en mmol/l x [0,309 x pH –0,469)] y gap del SID (SIDm-SIDe). Resultados: el pH pre-HD fue de 7,36 ± 0,08 y el pH post-HD de 7,44 ± 0,08 (p <0,001). No se apreciaron diferencias significativas entre pCO2 pre y post-HD. El HCO3 – y el exceso de bases se incrementaron durante la sesión (p <0,001). La SIDm descendió de manera significativa de 46,2 ± 2,9 preHD a 45 ± 2,3 post-HD (p <0,05). Por el contrario, la SIDe se elevó de 38,5 ± 3,8 a 42,9 ± 3,1 (p <0,001). El anion gap descendió de 18,6 ± 3,8 pre-HD a 12,8 ± 2,8 Eq/l post-HD (p <0,001) y el gap del SID de 7,6 ± 3 a 2,1 ± 2 (p <0,001). Se apreció una correlación entre el anion gap y el gap-SID tanto antes como después de la HDF. Asimismo, se apreció una correlación significativa entre el ¿ exceso de bases y ¿ del gap-SID. Conclusión: en conclusión, la aproximación físico-química de Stewart-Fencl no mejora la valoración del equilibrio ácido-base en pacientes en HDF crónica. En presencia de normocloremia la SIDm no refleja el proceso alcalinizante de la sesión de hemodiálisis. Bajo esta perspectiva, la sesión de hemodiálisis se concibe como una retirada de aniones inorgánicos no metabolizables, en especial el sulfato. El espacio dejado por estos aniones es reemplazado por OH–y secundariamente por HCO3–. La única ventaja vendría dada por una mejor valoración de los aniones no medidos mediante el gap del SID, sin el efecto de la albúmina y el fosfato.
INTRODUCTION
Metabolic acidosis is a common disorder in patients suffering renal failure on haemodialysis (HD).1 The evaluation of acid-base status is based on Henderson- Hasselbalch equation. With this equation, hydrogen-ion [H+] concentration behaviour is explained by variations of other variables such as HCO3, OH–, CO3H2, in an independent fashion. In 1983, P. Stewart developed an acid-base status quantitative analysis showing a system of independent variables that are modified independently of the rest.2 Amongst these variables included are: pCO2 that is regulated mainly through alveolar gas; the strong ion difference (SID), particularly the difference between the sum of strong cations (Na+, K+, Ca++, Mg++) and the sum of strong anions (Cl–, lactate); and total plasma concentration of non-volatile weak acids, mainly inorganic phosphate and albumin (A.Tot.). There is a second type of dependent variables modified as a group, simultaneously and only if one of the independent variables change. These include H+, OH– and HCO3 –. In 1992 Fencl, Figge et al. developed a mathematical model to estimate SID from pCO2, albumin and phosphate (SIDe). The difference between them (SIDm and SIDe) allows us to calculate the SID gap, which includes both metabolisable ions (pyruvate, acetoacetate, citrate, etc.) and nonmetabolisable ions (sulphate, hippurate) that take part in electro-neutrality. The aim of this study is to evaluate acid-base status in stable patients on haemodiafiltration (HDF) from both perspectives: classic (pH, HCO3, excess base and anion gap) and physical-chemical (SIDm, SIDe and SID gap).
MATERIAL AND METHODS
Thirty-five patients were studied, 24 male and 11 female, mean age of 67.2 ± 15.7 years and dry weight of 72.8 ± 19.8kg. They remained on HD for 68.3 ± 43.3 months. Seventeen patients (48.5%) were on “on-line” HDF and the rest on conventional HDF. The filter used was high-permeability 1.9m2 polysulfone. Session length was 253.6 ± 40.5 min. Blood flow (Qb) and dialysis liquid (Qb) were 368 ± 44 and 751 ± 83ml/min, respectively. A mean convection of 15.2 ± 6.4L was performed per session. The dialysis liquid employed had the following theoretical composition: Na 140, K 1.5, Cl 107, Mg 1 and acetate 4mEq/l. Calcium varied from 2.5 to 3.5mEq/l depending on patient’s needs. This same liquid was used as infusion liquid in “on-line” HDF and in conventional HDF a biocarbonate solution of 60mEq/l was used. The study was performed at the weekly intermediate session. Sample tests were taken from the pre-HD and post-HD arterial line (after having Qb at 50ml/min during 1 min.). From the samples, pH, pCO2, HCO3 –, albumin, Na+, K+, Cl– , Ca++, P, Mg and lactate were determined. pH, pCO2, HCO3 – determination, base excess, Ca++ and lactate were carried out in a gas analyser (IL GEM Premier 3000). Determination of Na, K, Cl, P, Mg and albumin was performed by laboratory standard techniques with an automatic auto-analyser (Olympus AV-640).
Definitions and calculations
SID measure (SIDm) was calculated as: [Na+] + [K+] + [Ca++] + [Mg++] – [Cl–] – [lactate]. Mg in mmol/l was converted to mEq/l multiplied by 2 and it was considered that 65% of total Mg corresponded to the ionised form. The estimated SID (SIDe) was determined from albumin by the formula proposed by Figge: 1.000 x 2.46–11 x pCO2/(10–pH) + Albumin g/dl x (0.123 x pH–0.631) + P in mmol/l x (0.309 x pH–0.469)3. From this formula albumin anionic (Album–) and phosphorus (P–) charges were calculated. The anion gap (AGap) was calculated by: (Na + K) – (Cl + HCO3–). The SID gap was calculated by: SIDm – SIDe. The variations in the session of base excess, albumin and phosphate charge, Cl and SID gap (ΔExBa, ΔAlbum.–, ΔP–, ΔCl– and Δgap-SID) were calculated as the difference between pre- and post-HDF values.
Statistical methods
The results are expressed as mean ± SD. Normality between variables was corroborated by Kolmogorov–Smirnov test. Student’s T test for paired data was used to analyse the difference between pre- and post HD variables. The correlation coefficients were determined by linear regression analysis by the least square method. Statistical signification was set at an error probability lower than 0.05 (p < 0.05).
RESULTS
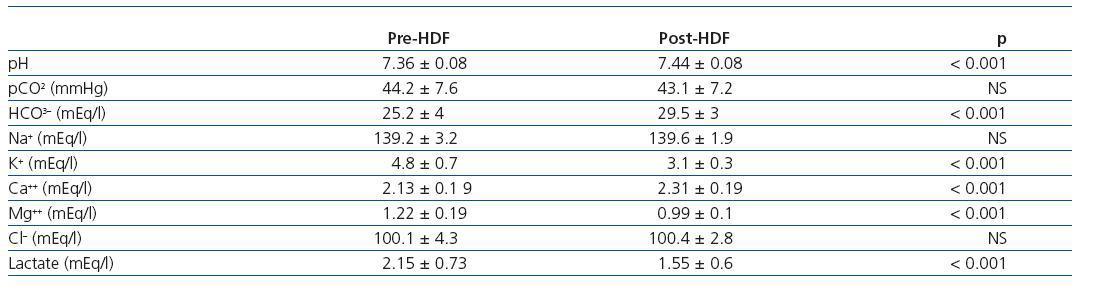
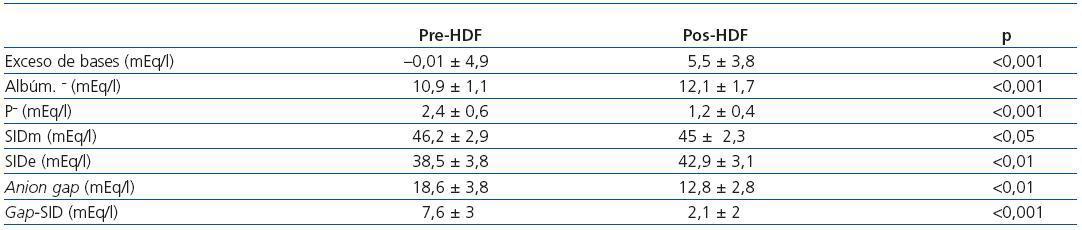
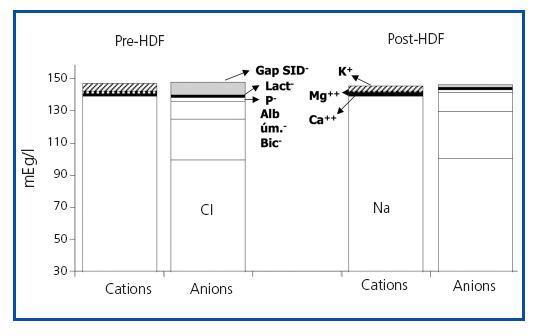
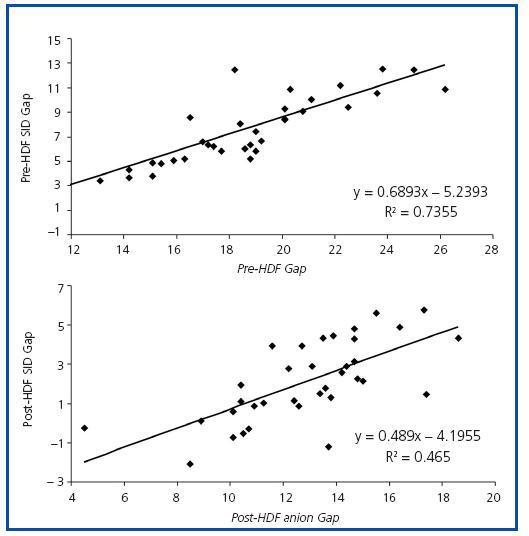
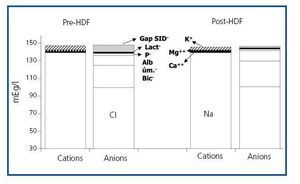
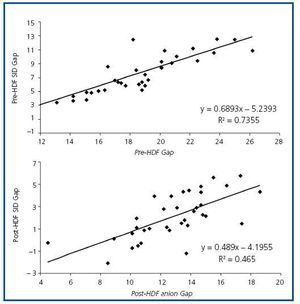
The values measured are expressed in table 1. pH, HCO3 and Ca++ increased significantly along the session, while K+, Mg++ and lactate values decreased. No significant differences were observed between pre- and post-HDF Na+ and Cl–. The values calculated are shown in table 2. A decrease in SIDm can be appreciated from 46.2 ± 2.9 to 45 ± 2.3mEq/l (p < 0.05), as well as in the anion gap, and SID gap, and in the anionic charge of P. On the contrary, SIDe increases from 38.5 ± 3.8 to 42.9 ± 3.1mEq/l (p < 0.01), as it does the albumin charge and base excess. Maintenance of plasma electro-neutrality between cations and anions pre- and post-HDF can be observed in figure 1. A correlation was observed between the anion gap and SIDgap pre- and post HDF, shown in figure 2. Furthermore, a significant correlation was observed between Δbase excess y ΔSID-gap (r = 0.45; p <0.01). No correlation was observed between Δbase excess and ΔAlbum.–, ΔP– and ΔCl–.
DISCUSSION
Metabolic acidosis is a common disorder in HD patients with renal failure. The main source of H+ in these patients comes from the metabolism of sulphur-containing amino acids, methionine, and cystine. Their oxidation4,5 generates sulphuric acid, a strong acid (low pK) that dissociates and increases the levels of sulphate and H+. From the classic approach viewpoint, bicarbonate is consumed by the generated H+. Therefore, the HD is seen as an alkalinising therapy transferring bicarbonate from the dialysis liquid into the blood to replenish the consumed bicarbonate. This results in an increase of pH, bicarbonate, and base excess at the end of the session.6 According to Stewart-Fencl physical-chemical approach, the strong ion difference (SIDm), specifically, i.e. the difference between measured strong cations and measured strong anions, is what controls the metabolic component of the acid-base status. In this way, metabolic acidosis and alkalosis are reflected in a decrease and increase of SID, respectively.7 Under this perspective, the patient on periodic HD evidences decreased SID in predialysis situation and the objective of the HD session is to increase reduced SID by the accumulation of other strong anions, such as sulphate, hippurate, etc.8 According to the Stewart-Fencl approach, since only independent variables can modify acid-base status, HD would not cause transfer of bicarbonate from dialysis liquid into blood, but it would eliminate the strong anions accumulated, thus normalising serum Cl and restoring SID, which translates into a secondary bicarbonate increase. This way bicarbonate stops being the main factor in the regeneration of acid-base status. In 1992 Fencl, Finge et al. developed a mathematical model to calculate SID when the other independent variables are known (pCO2, albumin and phosphate), as well as pH.3 This new definition of estimated SID (SIDe) is similar to the concept of buffer base (BB) developed by Singer and Hasting in 1948.9 The simplified equation is the one employed in our study, and it is composed of CO2T, anion charge of albumin, and anion charge of phosphate. The study by Leblanc et al. stresses the increment of SIDm during the session in patients on chronic HD.8 On the contrary, in our study we have observed a significantly slight decrease in SIDm during the HD session, moving from 46.2 to 45mEq/l by the end of the session. All this is in apparent contradiction with an increase of pH, bicarbonate, and base excess, which would increase as expected by the alkalinising effect of HD. We have confirmed similar results in patients on daily “online” HDF during a 1-year-follow-up period.10 How is this apparent contradiction possible between these two variables that are supposed to measure the same effect? As those stable patients on periodic HD usually have normal levels of Cl–, the not-measured strong anions (sulphate, hippurate, etc.) are the ones that decrease. These anions are not taken into account in the SIDm formula, so that, apparently, this variable will not change. On the contrary, SIDe will take into account that CO2T increases throughout the session. In our study we have only appreciated a significant correlation between Δbase excess and ΔSID-gap, but not between the first parameter and that of Δ Cl, Albumin– and P–, which makes clear that SID-gap is the only element associating with base excess regeneration. The study by Liborio et al.,11 with critically ill patients admitted to ICU, shows similar results to ours, but when classifying their patients in groups with high Cl– and low Cl– with relation to this anion levels in the dialysis fluid, they observed that acid-base status correction (by means of variation of base excess) is significantly higher in the group with high Cl– values. Furthermore, these authors observed a correlation between Δ base excess and Δ SID-gap and ΔCl–. In other studies the same authors emphasised the importance of Cl– serum levels as acidosis components in patients on chronic HD.12,13 This would corroborate our results, since, in this situation, the correction of acid-base status during the session would not only be due to the elimination of not-measured strong anions but also to a normalisation of Cl– serum levels. SIDm is only able to detect increments, whether when Na+ increases without accompanying Cl– or when Cl– decreases. Neither of these situations occurred in our patients included in the programme of chronic HDF. In HDF the composition of the re-infusion liquid with an increased SID would also accomplish the same objective. It is intended to use liquids with strong cations (Na+) with a smaller amount of strong accompanying anion (Cl–) in an attempt to restore SID.14 Other important aspect is the albumin and phosphate anion charges, included in the expression total weak anions (ATot). This physical-chemical approach considers the possibility that a decrease or an increase in these parameters may cause metabolic alkalosis or acidosis, respectively.15 In our study, the ATot as a whole were not modified throughout the session, as the increment of albumin charge post-session (10.9 to 12.1mEq/l) was compensated by the decrease in the phosphate charge (from 2.4 to 1.2mEq/l). The Stewart-Fencl approach allows evaluation of not measured anions much more precisely than the anion gap. This parameter largely used in the classic approach presents remarkable interferences on the part of serum albumin and phosphate, which make difficult the correct evaluation of the type of metabolic acidosis, mostly in situations with important alterations in the ATot components, such as nephrotic syndrome, malnutrition, negative protein catabolism or phosphorus intoxications.16,17 In fact, the difference between SIDm and SIDe provides a quantity of not-measured anions (gap-SID) free from the interference of weak acids and, therefore, partially dissociated. The studies of Kellum et al. make reference to a poor correlation scale between anion gap and gap-SID in critical patients at the ICU, with low concentrations of serum albumin.18 On the contrary, Gilfix et al. do find this correlation between AG and gap-SID with the same type of patients. Other studies have demonstrated the usefulness of this parameter in critical patients at the ICU.19 Our results do show an important correlation between anion gap and gap-SID, pre- and post-HDF, maybe due to the normal albumin figures throughout the session. Therefore the anion gap may be a good not-measured anion marker in stable chronic patients on HD, but its effectiveness is doubtful in patients with acute renal failure. However, it is gap-SID the parameter that best reflects not-measured anion accumulation during the inter-dialysis period. This parameter decreases significantly and, logically enough, it includes anions, especially sulphate, from protein catabolism, which will be adequately eliminated during the HDF session. As opposed to anion gap, gap-SID will not change either with pH or with the albumin and phosphate changes. To conclude, Stewart-Fencl approach does not improve the evaluation of acid-base status in patients on chronic HDF. In the presence of normochloraemia, SIDm does not reflect the alkalinising process of the haemodialysis session. Under this perspective, the HD session is conceived as a nonmetabolisable inorganic anion withdrawal, especially sulphate. The room left by these anions is replaced by OH– and secondarily by HCO3 –. The only advantage would involve a better evaluation of anions not measured by gap- SID, without the effect of albumin and phosphate.
Table 1. Measured parameters of acid-base status pre- and post-HDF
Table 2. Measured parameters of acid-base status pre- and post-HDF
Figure 1. Plasma electro-neutrality between pre- and post-HDF cations and anions.
Figure 2. Correlation between the levels of anion gap and SID gap pre- and post-HDF.