Metabolic acidosis (MA) is a common complication of chronic kidney disease (CKD) and is associated with numerous adverse effects, which is why its correction is highly recommended. Oral sodium bicarbonate is the current treatment of choice.
ObjectivesTo describe the prevalence of MA in advanced CKD patients and to determine the clinical and biochemical characteristics associated with its successful correction.
Material and methodsRetrospective, observational cohort study in adult patients with CKD stage 4–5. The inclusion criteria were: not being treated with alkali therapy at the time of inclusion, and to have at least three consecutive glomerular filtration rate (GFR) measurements and biochemical parameters during a minimum follow-up period of 3 months. Incident patients with serum bicarbonate < 22 mEq / l were included in the follow-up study and treated with oral sodium bicarbonate. Correction was considered successful when more than half of the samples and the mean bicarbonate levels during individual follow-up were ≥ 22 mEq / l.
ResultsThe study group consisted of 969 patients (age 65 ± 14 years, 507 males) with a mean GFR of 14.8 ± 4.5 mL / min / 1.73 m 2. At baseline, 530 patients (55%) had serum bicarbonate < 22 mEq / l. They were treated with sodium bicarbonate and followed for 15 months. Satisfactory correction of MA was only achieved in 133 patients (25%). By multivariate logistic regression analysis, the main characteristics of patients with adequate control of MA were: age (OR = 1.03; 95% CI 1.01–1.05), baseline GFR (OR = 1.07; 1.02–1.12), and treatment with proton-pump inhibitors (OR = 1.61; 95% CI 1.06–2.44). Patients who achieved successful correction of MA showed slower CKD progression (-1.67 ± 3.71 vs -4.36 ± 4.56 ml / min / 1.73 m 2 / year, P < 0.0001), and lower average serum potassium concentration (5.1 ± 0.5 vs 5.3 ± 0.5, P < .0001) than those who did not. However, there were no differences in the hospitalization or mortality rate.
ConclusionMA is a common complication of advanced CKD but difficult to manage with current therapies. Due to the significant potential benefit of controlling MA, new, more effective therapies should be further researched.
La acidosis metabólica (AM) es una alteración frecuente en la enfermedad renal crónica (ERC) que se asocia a numerosas complicaciones, por lo que su corrección es recomendable. El bicarbonato sódico oral es actualmente el tratamiento de elección.
ObjetivosDescribir la prevalencia de AM en la ERC avanzada, y determinar cuáles son las características clínicas y bioquímicas que se asocian a una corrección adecuada.
Material y métodosEstudio retrospectivo de observación en una cohorte de pacientes adultos con ERC estadio 4-5. Los criterios de inclusión fueron: no estar siendo tratado con alcalinos en el momento de la inclusión, y tener al menos 3 medidas consecutivas de filtrado glomerular (FG) y parámetros bioquímicos durante un periodo > 3 meses. Los pacientes incidentes con un bicarbonato sérico < 22 meq/l se incluyeron en estudio de seguimiento, siendo tratados con bicarbonato sódico oral. Se consideró que la corrección fue adecuada cuando más de la mitad de las muestras, y la media de los niveles de bicarbonato durante el seguimiento individual fueron ≥ 22 meq/l.
ResultadosSe incluyeron 969 pacientes (edad 65 ± 14 años, 507 hombres) con filtrado glomerular (FG) medio 14,8 ± 4,5 ml/min/1,73 m2. Basalmente, 530 pacientes (55%) que mostraron un bicarbonato sérico < 22 meq/l, fueron tratados con bicarbonato sódico y seguidos durante 15 meses. En tan solo 133 pacientes (25%) se alcanzó una corrección satisfactoria de la AM. Por regresión logística multivariable las principales características de los que se logró el control adecuado de la AM fueron: edad (OR = 1,03; I.C.95%1,01 – 1,05), FG basal (OR = 1,07; 1,02 – 1,12), y tratamiento con inhibidores de bomba protones (OR = 1,61; I.C.95%1,06 – 2,44). En aquellos en los que se logró corrección de AM tuvieron progresión más lenta de ERC (-1,67 ± 3,71 vs. -4,36 ± 4,56 ml/min/1,73 m2/año, p < 0,0001) y menor concentración de potasio sérico promedio (5,1 ± 0,5 vs. 5,3 ± 0,5, p < 0,0001) que los del resto de pacientes, aunque no se observaron diferencias ni en ni en la tasa de ingresos hospitalarios y mortalidad.
ConclusiónLa AM es una alteración frecuente en la ERC avanzada, pero de difícil corrección con los tratamientos actuales. Debido al importante beneficio que puede suponer el control de la AM, se deberían investigar nuevas terapias más efectivas.
Metabolic acidosis (MA) is frequent in patients chronic kidney disease (CKD).1–3 The deterioration of the renal function causes a reduction in the net excretion of acids resulting a positive balance of hydrogen ions. When the glomerular filtration falls below 20−25 ml / min, there is a reduction of bicarbonate in the blood.1–3 With more sensitive biochemical analysis, such as urinary excretion of ammonium1,2,4 or citrate,5 it can be shown that this metabolic defect begins in earlier stages of CKD and even before a reduction serum bicarbonate is documented.
The negative effects of MA on CKD have been the subject of numerous studies showing its association with abnormalities in bone,6,7 metabolic and inflammatory disorders,8,9 endocrine alteration,10–12 progression of CKD13–18 and mortality.19–21 Interestingly, correction of MA improves or reverses many of these abnormalities22–27 which support the recommendation of an active treatment of MA.
The treatment of MA associated with CKD before the need for dialysis has been based on providing alkalis, either through the diet (fruits and vegetables),28 administration of bicarbonate salts29,30 and reduction of the dietary intake of acids (reduction of protein intake).31
Oral sodium bicarbonate is currently the treatment of choice due to its availability, tolerance and absence of serious adverse effects29,30; although not expensive medicine, it is not being financed by health services, which increases the risk of nonadherence to the treatment.
The objectives of the present study were to analyze the prevalence of MA in advanced CKD, and to determine the factors associated with its inadequate or incomplete correction despite active attempt to treat this metabolic alteration. The results of this study could help to improve the models of alkaline prescription in CKD.
Material and methodsRetrospective longitudinal observational study in a cohort of adult patients diagnosed with CKD stages 4–5 not on dialysis, followed up in the advanced CKD outpatient clinic (ACKD clinic) during the period from January 2000 to December 2016. The selection criteria were: not being treated with alkalis at the time of inclusion, or with certain specific drugs (eg sevelamer hydrochloride) or clinical situations that could worsen MA (hemodynamic instability, lactic acidosis, urinary tract obstruction, etc.), being followed in the ACKD clinic for more than 3 months and have carried out during this time at least 3 measurements of renal function, serum bicarbonate and biochemical parameters of interest.
All patients were referred to the ERCA consultation for progressive deterioration of kidney function. Demographic and clinical data, and information on prescribed medication was obtained from medical records as well as physical examination, and history. Comorbidity was assessed at the time of inclusion, using the Davies index,32 and patients were categorized into 3 groups: no comorbidity, mild-moderate, and severe.
All samples and biochemical analysis were processed in the same central laboratory by conventional methods (Advia Chemistry Autoanalyzer, Siemens Healthcare Diagnostics, New York, USA), using fresh samples (not stored), and both calibrations and Creatinine traceability was performed according to the recommendations of international NKDEP standards.33 Glomerular filtration was estimated using the abbreviated formula MDRD.34 The determination of serum bicarbonate was performed in less than 15 min from the extraction of the venous sample, using a gasometric analyzer (ABL800 FLEX, Radiometer Ibérica, Spain).
Patients were followed-up regularly, with visits to the outpatient clinic every 30–90 days. To determine the rate of CKD progression in each patient, a linear regression was performed between the glomerular filtration rate (GFR) estimated in each control and the time elapsed since the first appointment, with a precision of days. The resulting slope of this linear equation was expressed in ± ml / min / 1.73 m 2 / year; negative or positive values of this parameter meant a progression of renal failure or recovery of renal function, respectively.
All patients who presented a basal serum bicarbonate < 22 mEq / l were prescribed a diet with reduction of animal protein, without restriction of fruits and vegetables, and oral sodium bicarbonate with doses that were adjusted trying to maintain a serum bicarbonate equal to greater than 22 mEq / l or less than 29 mEq / l, according to the recommendations of the National Kidney Foundation35 and KDIGO.36 The average dose of oral sodium bicarbonate was recorded in every visit and it was expressed as total daily amount taken adjusted for body weight.
Study design and statistical methodsRetrospective longitudinal observation study in a cohort of patients with advanced CKD. In each patient, all measurements of serum bicarbonate obtained during their follow-up were collected.,.
Those patients who at baseline had serum bicarbonate < 22 mEq / l were included in the longitudinal study.
The correction of bicarbonate was considered adequate if more than half of the sample measurements, and the average bicarbonate levels during the individual follow-up period were ≥ 22 mEq / l. Additionally, there were also analyzed the determinants of a less strict MA correction defined as an average serum bicarbonate during the follow-up period ≥ 20 mEq / l.
The differences observed between patients with or without control of MA were analyzed using multiple logistic regression. In these two groups there were also compared the progression of CKD (slope of glomerular filtration / time ratio), mortality rate and hospital admissions.
Parametric or non-parametric tests were used for the descriptive comparison of the continuous variables, depending on their characteristics; Chi-square test was used for the categorical variables.
Descriptive statistical data is presented as mean and standard deviation, or as median and interquartile ranges (IQ) for continuous variables, and as percentages for categorical variables. A p < 0.05 was considered statistically significant, and all p values shown are bilateral. Statistical analyzes were performed with IBM SPSS Statistics 24.0 software (IBM Corp. Armonk, USA).
ResultsThe total study group included 969 patients (age 65 ± 14 years, 507 men) with a mean GFR of 14.8 ± 4.5 ml / min / 1.73 m 2. Table 1 shows the characteristics of the total group and those patients that initially presented with or without MA.
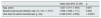
Baseline characteristics of the total study group and of those with or without initial metabolic acidosis.
| Total group N = 969 | Without initial acidosis N = 439 (45%) | With initial acidosis N = 530 (55%) | p* | |
|---|---|---|---|---|
| Age, years (± SD) | 65 (14) | 67 (13) | 64 (14) | 0.006 |
| Gender, man / woman | 507/462 | 230/209 | 277/253 | 0.968 |
| Comorbidity index (0, 1, 2) | 378/492/99 | 147/229/63 | 231/263/36 | < 0.0001 |
| Diabetes,% | 37 | 43 | 32 | < 0.0001 |
| Body mass index, kg / m2 | 29.6 (5.9) | 29.8 (5.7) | 29.4 (6.1) | 0.263 |
| Systolic blood pressure, mm Hg | 158 (27) | 158 (26) | 158 (28) | 0.701 |
| Diastolic blood pressure, mm Hg | 87 (14) | 86 (14) | 88 (14) | 0.130 |
| Serum bicarbonate, mEq / l | 21.6 (3.7) | 24.8 (2.2) | 18.9 (2.2) | < 0.0001 |
| Glomerular filtration rate, ml / min / 1.73 m2 | 14.8 (4.5) | 15.9 (4.6) | 13.9 (4.2) | < 0.0001 |
| Serum sodium, mEq / l | 141 (3) | 141 (3.2) | 140 (3.2) | 0.297 |
| Serum potassium, mEq / l | 5.1 (0.7) | 4.9 (0.6) | 5.2 (0.8) | < 0.0001 |
| Serum chlorine, mEq / l | 106 (8) | 104 (8) | 108 (6) | < 0.0001 |
| Anion Gap, mEq / l | 18 (4) | 16 (3) | 19 (4) | < 0.0001 |
| Hemoglobin, g / dl | 11.5 (5.4) | 11.9 (5.4) | 11.3 (5.5) | 0.083 |
| Proteinuria, g / g creatinine | 2.09 (2.39) | 2.14 (2.62) | 2.06 (2.19) | 0.637 |
| Calcium salts,% | 59 | 51 | 66 | < 0.0001 |
| Diuretics,% | 65 | 72 | 59 | < 0.0001 |
| ACEI / ARA,% | 76 | 75 | 77 | 0.510 |
| Beta blockers,% | 25 | 29 | 23 | 0.038 |
| Calcium antagonists,% | 48 | 49 | 48 | 0.710 |
| Proton-pump inhibitor, % | 40 | 43 | 37 | 0.047 |
ARA: angiotensin receptor antagonists; ACEI: angiotensin-converting enzyme inhibitors.
530 patients (55%), showed MA from the beginning. The main characteristics of these patients, compared with those who did not present it, were: younger, lower comorbidity and diabetes, lower GFR, higher serum potassium concentration and lower frequency of diuretic treatment (Table 1).
These 530 patients with initial MA were treated with oral sodium bicarbonate and formed the longitudinal follow-up study group.
The median follow-up period was 443 days (IQ ranges: 233–818 days), and the number of total serum bicarbonate samples analyzed during this period was 4,899, with a median of 7 (IQ ranges: 5–12) samples per patient.
The average dose of oral sodium bicarbonate prescribed in the group of patients was 1813 ± 1004 mg / day (21.6 ± 11.9 mEq / day), or 24.8 ± 14.7 mg / kg body weight / day ( 0.29 ± 0.17 mEq / kg / day).
In only 133 patients (25%) was the objective of adequate correction of MA achieved. The main characteristics of these patients, compared with those of the rest of the study group, are shown in the Table 2.
Characteristics of patients with metabolic acidosis treated and with adequate or inadequate response.
| Adequate response N = 133 (25%) | Inadequate response N = 397 (75%) | p* | |
|---|---|---|---|
| Age, years | 69 (13) | 63 (15) | < 0.0001 |
| Gender, man / woman | 64/69 | 213/184 | 0.269 |
| Comorbidity, (0, 1, 2) | 45/78/10 | 186/185/26 | 0.031 |
| Diabetes,% | 34 | 31 | 0.541 |
| Body mass index, kg / m2 | 29.5 (6.3) | 29.4 (6.0) | 0.857 |
| Systolic blood pressure, mm Hg | 157 (30) | 159 (27) | 0.447 |
| Diastolic blood pressure, mm Hg | 86 (15) | 88 (14) | 0.136 |
| Initial serum bicarbonate, mEq / l | 19.4 (1.9) | 18.8 (2.3) | 0.006 |
| Average bicarbonate, mEq / l | 23.4 (1.2) | 20.2 (1.4) | < 0.0001 |
| Initial glomerular filtration rate, ml / min / 1.73 m2 | 14.9 (3.9) | 13.6 (4.3) | 0.002 |
| Initial serum sodium, mEq / l | 140 (3) | 140 (3) | 0.857 |
| Initial serum potassium, mEq / l | 5.1 (0.6) | 5.2 (0.7) | 0.129 |
| Time-average serum potassium, mEq / l | 5.1 (0.5) | 5.3 (0.5) | < 0.0001 |
| Initial serum chlorine, mEq / l | 107 (10) | 108 (4) | 0.022 |
| Initial anion gap, mEq / l | 18 (4) | 19 (4) | 0.558 |
| Hemoglobin, g / dl | 11.1 (1.6) | 11.4 (6.2) | 0.630 |
| Proteinuria, g / g creatinine | 1.756 (2.171) | 2169 (2.187) | 0.059 |
| Average dose of oral sodium bicarbonate, mg / day | 1296 (776) | 1987 (1013) | < 0.0001 |
| Average dose of weight-adjusted oral sodium bicarbonate, mg / kg / day | 18 (12) | 27 (15) | < 0.0001 |
| Treatment with calcium salts,% | 65 | 67 | 0.699 |
| Diuretics,% | 62 | 58 | 0.514 |
| ACEI / ARA,% | 74 | 78 | 0.420 |
| Beta blockers,% | 25 | 22 | 0.490 |
| Calcium antagonists,% | 44 | 48 | 0.395 |
| Proton-pump inhibitor, % | 49 | 33 | 0.001 |
ARA: angiotensin receptor antagonists; ACEI: angiotensin-converting enzyme inhibitors.
The patients in whom MA was corrected were older, had better residual renal function, but paradoxically the prescribed doses of oral sodium bicarbonate were lower (Table 2 ). Also patients with better MA control were treated more frequently with proton pump inhibitors. No other significant differences were observed.
Multivariate logistic regression analysis, without including oral sodium bicarbonate doses as independent variable due to their negative relationship with the dependent variable, showed that the best determinants of adequate correction of MA were: age, baseline GFR, and treatment with Proton-pump inhibitor (Table 3).
Logistic regression on the determinants of adequate correction of metabolic acidosis.
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Odds ratio (95% CI OR) | p | Odds ratio (95% CI OR) | p | |
| Age, years | 1.028 (1.011−1.045) | 0.001 | 1.030 (1.013−1.046) | < 0.0001 |
| Gender, male = 1 | 0.744 (0.483−1.144) | 0.178 | ||
| Basal glomerular filtration rate, ml / min / 1.73 m2 | 1.076 (1.025−1.130) | 0.003 | 1.072 (1.023−1.124) | 0.004 |
| Comorbidity index, 0, 1, 2 | 1.110 (0.729−1.693) | 0.626 | ||
| Diabetes mellitus, 0, 1 | 0.858 (0.503−1.462) | 0.573 | ||
| Body mass index, kg / m2 | 0.995 (0.959−1.034) | 0.810 | ||
| Initial serum bicarbonate, mEq / l | 1.108 (0.998−1.231) | 0.055 | ||
| Diuretic, 0, 1 | 1.050 (0.674−1.635) | 0.830 | ||
| ACEI / ARA, 0, 1 | 0.848 (0.511−1.406) | 0.523 | ||
| Beta Blocker, 0, 1 | 1.089 (0.653−1.813) | 0.745 | ||
| Calcium antagonist, 0, 1 | 0.819 (0.540−1.241) | 0.346 | ||
| Proton pump inhibitor, 0, 1 | 1.626 (1.049−2.523) | 0.030 | 1.607 (1.061−2.435) | < 0.0001 |
ARA: angiotensin receptor antagonists; ACEI: angiotensin-converting enzyme inhibitors.
If the oral sodium bicarbonate dose is introduced as a dependent variable, this turned out to be one of the main determinants, although negative, of a satisfactory correction of MA, to the detriment of treatment with proton pump inhibitors (Table 4).
Logistic regression; determinants of a satisfactory correction of metabolic acidosis by entering the prescribed dose of oral sodium bicarbonate as a dependent variable.
| Odds ratio (95% CI OR) | p | |
|---|---|---|
| Age, years | 1.027 (1.011−1.044) | 0.001 |
| Baseline glomerular filtration rate, ml / min / 1.73 m2 | 1.058 (1.008−1.111) | 0.022 |
| Oral dose of sodium bicarbonate, g / day | 0.455 (0.351−0.590) | < 0.0001 |
Out of the best predictive equation: proton pump inhibitors (OR: 1,461, 95 % CI: 0.944–2.261; p = 0.089).
Throughout the follow-up period, the correction of MA ( mean plasma bicarbonate concentration ≥ 20 mEq/l) was achieved in 385 patients (73%). Multivariate logistic regression revealed that the main determinants of this outcome were initial serum bicarbonate (OR: 1.315; 95% CI: 1.200-1.442; p < 0.0001) and treatment with proton pump inhibitors (OR: 1683; CI. 95%: 1,091−2,595; p = 0.019), and neither age nor initial kidney function were determinans in the best prediction equation.
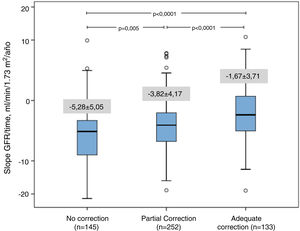
The correction MA was associated with slower CKD progression (Table 5); there were significant differences in the progression (slope of the glomerular filtration / time) according to the degrees of correction of MA (Fig. 1)
Outcome variables according to the degree of correction of metabolic acidosis.
| Proper correctiona | Without proper correction | p | Partial correctionb | No partial correction | p | |
|---|---|---|---|---|---|---|
| No. of patients, (%) | 133 (25) | 397 (75) | 385 (73) | 145 (27) | ||
| Slope Glomerular filtration, rate ml/min / 1.73 m 2 / year | −1.676 ± 3.711 | –4.352 ± 4.563 | < 0.0001 | –3.077 ± 4.142 | –5.282 ± 5.052 | < 0.0001 |
| Hospital admissions; days / year | 3.01 ± 5.43 | 2.98 ± 6.61 | 0.957 | 2.77 ± 5.56 | 3.56 ± 8.01 | 0.198 |
| Annual mortality rate, patient-years | 13.6 | 12.6 | 0.250 | 12.4 | 15.6 | 0.312 |
By contrast, no significant differences were observed in the hospital admission rate or in the mortality (Table 5).
DiscussionThe results of this study show that MA has a high prevalence in advanced CKD (55%), and that an appropriate correction with oral sodium bicarbonate is not easily achieved. Adequate control of MA was accomplished in only 25% of the treated patients; a control of MA was defined by a serum bicarbonate greater than or equal to 22 mEq / l. However, mean serum bicarbonate levels higher than 20 mEq / l were achieved in more than 70% of the treated patients.
Numerous studies have shown the adverse effects in relation with CKD-associated MA.6–21 The correction of MA has been estimated as adequate, by consensus, if serum bicarbonate concentrations reach and are maintained equal or above 22 mEq / l 35,36 although overcorrection has been associated with cardiovascular risks.13
The oral sodium bicarbonate is currently the treatment of choice for MA of CKD patients.29,30 Although inexpensive and safe, the results of the present study show that the real efficacy of this treatment was below the expectations. Based on some results of this study, some reasons for this poor response could be contemplated, such as: a lack of adherence to treatment and / or a poor pharmacological availability of sodium bicarbonate for oral administration.
The negative relationship between the prescribed dose and the probability of adequate control of AM may suggest a non-compliance with the prescribed treatment. Oral sodium bicarbonate is generally well tolerated, although adverse gastrointestinal effects are not uncommon: bloating, meteorism, or complaints of bad taste of the medication when taken as diluted powder.29 In addition, this medication is not covered by the different regional health administration in the country, which hinders its correct compliance by a population not used to the disbursement, even of a small amount, of pharmacological treatments.
The mean dose of sodium bicarbonate was approximately 1800 mg per day (0.3 mEq / kg of body weight). Prescribing higher doses of oral sodium bicarbonate did not improve the results.
In an experimental study on the efficacy of oral sodium bicarbonate, in a small group of patients with CKD and mild acidosis, it was shown that the increase in serum bicarbonate after 2 weeks of treatment correlated with the total amount of oral sodium bicarbonate prescribed.23 In our patients the absence of a positive correlation between the dose of oral sodium bicarbonate prescribed and the correction AM reinforces the hypothesis of a possible lack of adherence as a cause of the observed therapeutic failure. Another alternative and / or complementary explanation could be a poor pharmacological availability of oral sodium bicarbonate. In the present study there was an association between the use of proton pump inhibitors and a better correction of MA suggesting an improved better pharmacological availability of oral sodium bicarbonate.
When a patient ingest oral sodium bicarbonate, there is a rapid chemical reaction with hydrochloric acid in the stomach, which results in the production of H2O, CO2 and ClNa.37 Excess non-neutralized bicarbonate in the stomach passes into the small intestine where it is absorbed. The increase in gastric pH stimulates parietal cells to secrete more ClH into the gastric lumen. This forced secretion of ClH leads to the formation and passage of bicarbonate to the perigastric capillaries and the systemic circulation. This abrupt increase in bicarbonate production may exceed the maximum tubular reabsorption capacity and it is eliminated in the urine.37 Thus, the administration proton pump inhibitors to block stomach H + production may reduce the impact of these reactions, facilitating the absorption of bicarbonate in the small intestine and preventing a loss of urinary bicarbonate. Inhibition of gastric acidity could also improve tolerance and treatment compliance by reducing gastric gas (CO 2 ) formation a main cause of adverse gastrointestinal effects of this medication.29
Nevertheless, we do not believe that proton pump inhibitors should be indicated as specific adjuvants in the treatment of MA. Instead, we believe that galenic forms of sodium bicarbonate that could prevent the reaction with gastric acid, with slower and more sustained release throughout the gastrointestinal tract, would improve the tolerance and availability of the prescribed doses.
Another alternative to sodium bicarbonate could be citrate salts26,38,39 which although probably better tolerated, have the problem of increasing intestinal absorption of aluminum.40 Clinal studies are evaluating a chlorine and hydrogenation in the gastric lumen (insoluble polymer TRC101),41 with effects that mimics the metabolic consequences of vomiting (negative balance of chlorine and hydrogen), which causes significant increases in serum bicarbonate levels in patients with CKD, with the advantage of not providing any cation.
As in numerous previous studies,24–28 adequate, and even incomplete, correction of MA in our patients was associated with slower progression of CKD, although due to the study design it cannot be establish the causality of this association. A significantly lower mean serum potassium concentration was also observed in patients with adequately corrected MD as compared to the rest of patients. Although the pathogenic relationship between hyperkalemia and MA in CKD may be reciprocal,42 the results of this study would indicate the benefit of adequate correction of MA on the control of serum potassium in CKD.
This study has limitations. Due to the retrospective design, firm causal relationships cannot be established, and since it was performed in a single center, with some specific treatment criteria, the results may not be generalized.
The adherence was not analyzed rigorously by monitoring medication consumption or request for prescriptions, therefore the lack of adherence should be considered as assumption based on the indirect findings of the study and the daily information provided by the patients. The differences in the efficacy of oral sodium bicarbonate according to the pharmacological forms used (powder for solution, tablets or capsules) were not analyzed.
In conclusion, MA is a very frequent alteration in advanced CKD, that is difficult to correct with current treatments. Due to the important benefits of an adequate correction of MA, new forms of treatment should be investigated, including dietary recommendations, that allow for greater efficacy and adherence.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Caravaca-Fontán F, Díaz-Campillejo R, Valladares J, Arnaldo CL, Barroso S, Luna E, et al. Acidosis metabólica en la enfermedad renal crónica:dificultades para una corrección adecuada. Nefrologia. 2020;40:328–335.