Long non-coding RNAs (lncRNAs) have garnered interest because of their roles in cancer progression. We aimed to explore the role of the lncRNA embigin pseudogene 1 (EMBP1)-miR-9-5p axis in renal cell carcinoma (RCC).
Materials and methodsExpression profiling of miR-9-5p and EMBP1 were performed in RCC cell lines and tumor samples. To evaluate miR-9-5p and EMBP1's role in proliferation, invasion, migration, and colony formation, we performed in vitro assays along with studies in a xenograft tumor model. In silico binding site analysis using the RNA22 algorithm, RNA-immunoprecipitation (RIP), and luciferase reporter assays were used to validate a direct interaction between EMBP1 and miR-9-5p. Changes in key proteins were also analyzed.
ResultsmiR-9-5p was significantly down-regulated, and EMBP1 was significantly up-regulated, in RCC cell lines and tumor tissue. The clinicopathological characteristics of RCC patients significantly correlated with their expression. Overexpression of miR-9-5p or EMBP1 suppression in RCC cell lines significantly retarded their proliferative, migratory, and invasive behavior, in addition to promoting apoptosis and cell-cycle arrest. EMBP1 directly binds to and negatively regulates miR-9-5p. The EMBP1-miR-9-5p axis dysregulated the expression of the epithelial-to-mesenchymal transition (EMT) markers E-cadherin, claudin, and vimentin, the stemness markers KLF4 and Nanog, and the cell cycle checkpoint gene cyclin E2 (CCNE2) and its downstream mediator E2F1. miR-9-5p overexpression or EMBP1 suppression inhibited xenograft tumor growth in vivo, effects that were abrogated by CCNE2 overexpression.
ConclusionsOur findings suggest an important role of the EMBP1/miR-9-5p axis dysregulation in RCC tumor progression.
Los ARN no codificantes largos (ARNncl) han recibido interés debido a sus diversos roles en la progresión del cáncer. Nuestro objetivo era explorar el rol del eje ARNncl pseudogén embigin 1 (EMBP1)-miR-9-5p en el carcinoma de células renales (CCR).
Materiales y métodosLa descripción de la expresión de miR-9-5p y EMBP1 se realizó en líneas de células del CCR y muestras tumorales. Para evaluar el rol de miR-9-5p y EMBP1 en la proliferación, la invasión, la migración y la formación de colonias, realizamos ensayos in vitro junto con estudios en un modelo de tumor de xenotrasplante. Se utilizaron análisis del lugar de unión in silico mediante el algoritmo RNA22, inmunoprecipitación de ARN (RIP) y ensayos de luciferasa para validar una interacción directa entre EMBP1 y miR-9-5p. También se analizaron los cambios en las principales proteínas.
ResultadosEl compuesto miR-9-5p se reguló a la baja significativamente y EMBP1 se reguló al alza significativamente, en las líneas de células del CCR y en el tejido tumoral. Las características clinicopatológicas de los pacientes con CCR estaban significativamente relacionadas con su expresión. La sobreexpresión de miR-9-5p o la supresión de EMBP1 en las líneas de células del CCR retrasó significativamente su comportamiento proliferativo, migratorio e invasivo, además de favorecer la apoptosis y la detención del ciclo celular. EMBP1 se une directamente a miR-9-5p y lo regula negativamente. El eje EMBP1/miR-9-5p desreguló la expresión de los marcadores de la transición epitelio-mesénquima (TEM), E-cadherina, claudina y vimentina, los marcadores de troncalidad KLF4 y Nanog, y el gen de punto de control del ciclo celular ciclina E2 (CCNE2) y su mediador descendente E2F1. La sobreexpresión de miR-9-5p o la supresión de EMBP1 inhibió el crecimiento del tumor de xenotrasplante in vivo, efectos que fueron invalidados por la sobreexpresión de CCNE2.
ConclusionesNuestros hallazgos sugieren un rol importante de la desregulación del eje EMBP1/miR-9-5p en la progresión tumoral del CCR.
Renal cell carcinoma (RCC) is the most prevalent kidney malignancy in adults and represents almost 90% of all the kidney cancer cases reported.1 RCC is also one of the primary causes of cancer-related mortality worldwide.2,3 With an increased prevalence over the years, RCC accounts for approximately 3–4% of all malignancies among adults in the United States, with roughly 64,000 new cases diagnosed and 14,000 deaths reported yearly.3 Three are three major histological subtypes- clear cell RCC (ccRCC), constituting 70–80% of all RCC malignancies, papillary RCC (pRCC), comprising 10–15%, and only 5–10% comprise of chromophobe RCC (chRCC). The most common subtype, ccRCC, is usually diagnosed at an advanced stage of metastatic disease, and thus, has a reduced five-year survival.4 Advanced and aggressive RCC cases have a poor prognosis and are usually resistant to traditional therapies like chemotherapy and hormone therapy5; thus, with limited therapeutic options, these RCC cases have a high rate of recurrence and mortality.
The aggressiveness of epithelial cell-origin cancers are greatly associated with the epithelial-to-mesenchymal transition (EMT).6 EMT enhances cell stemness, migration, and invasion properties and inhibits cell senescence and apoptosis. The dysfunction of epithelial cell-to-cell adhesion and acquisition of the mesenchymal phenotype and mesenchymal cell markers during initiation of migration and invasion marks the most crucial step of EMT. Clinically, EMT maintains (CSC) phenotypic characteristics that have been associated with chemoresistance and recurrence.7 Thus, understanding the signaling mechanism(s) controlling EMT progression in RCC cells is crucial in developing effective therapeutics for aggressive RCCs.
Non-coding RNAs (ncRNAs), such as long non-coding RNA (lncRNA) and microRNAs (miRNA), have garnered interest in last few decades because of their crucial role in cancer progression.8,9 But, the regulatory interactions among these ncRNAs and their biological implications in cancer cells remain unexplored. The lncRNA embigin pseudogene 1 (EMBP1; chr1:121,260,910–121,313,686+ (GRCh37/hg19)) has been reported to be upregulated in RCC tumors and is hypothesized to be a predictor of poor prognosis and metastasis in RCC patients.10 The miRNA miR-9-5p, which is transcribed from the human chromosome loci 1q22, 5q14.3, and 15q26.1,11,12 is known to be dysregulated in several malignancies and can act as a tumor-suppressive miRNA13–16 or a tumorigenic miRNA.17,18 Notably, a recent bioinformatics analysis has identified a putative EMBP1/miR-9-5p/CCNE2 axis in RCC tumors that is statistically associated with RCC patient survival.10
Despite this evidence, the roles and relationship between EMBP1 and miR-9-5p in RCC remain uncharacterized. We found EMBP1 and miR-9-5p to be significantly upregulated and downregulated, respectively, in RCC cells as compared with their respective controls. Their expressions were also associated with key clinicopathological characteristics. Further analysis revealed a direct and sequence-specific binding of EMBP1 to miR-9-5p, which regulates miR-9-5p's activity. Subsequent analysis revealed that overexpressing miR-9-5p reduced proliferative, migratory, and invasive characteristics and increased cell-cycle arrest and apoptosis. Similar functional effects were seen by silencing of EMBP1 expression. Analysis of their role in EMT revealed that the miR-9-5p overexpression or EMBP1 silencing induced the expression of the epithelial marker proteins E-cadherin and claudin while reducing expression of the mesenchymal marker vimentin and the stemness markers KLF4 and Nanog. A concomitant decrease was also seen in the cell cycle checkpoint gene cyclin E2 (CCNE2) and its downstream effector E2F transcription factor 1 (E2F1) following miR-9-5p overexpression or EMBP1 silencing. Finally, our in vivo study using murine xenograft tumors showed that miR-9-5p overexpression or EMBP1 suppression produced an inhibition in tumor growth, effects that were abrogated by CCNE2 overexpression. Thus, our findings suggest an important role of the EMBP1/miR-9-5p axis dysregulation in RCC tumor progression.
MethodsThe study was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University. All human tissue used in this study had been obtained following written informed consent from the donors. The experimental methods are fully detailed in the Supplementary Information.
ResultsMiR-9-5p is down-regulated, and EMBP1 is up-regulated, in RCCReal-time quantitative PCR (RT-qPCR) analysis revealed significantly reduced expression of miR-9-5p (Supp. Fig. 1A) and significantly higher EMBP1 expression (Supp. Fig. 1C) in the ACHN and Caki-1 RCC cell lines as compared to the non-malignant renal epithelial cell line HK-2 (Ct values: Supp. Tables 1 and 2). These findings were mirrored in RCC tumor tissue samples (n=65) relative to matching normal kidney tissue samples (Supp. Fig. 1B and 1D, Ct values: Supp. Tables 3 and 4).
Clinical significance of miR-9-5p and EMBP1 in RCCTo evaluate the clinical salience of miR-9-5p and EMBP1 expression, we analyzed if their expression was statistically associated with the Fuhrman grade (the grading system widely used to classify RCC)19 and tumor stage (pT). miR-9-5p and EMBP1 expression both correlated with these clinicopathological characteristics in our sample cohort. Patients with low tumor miR-9-5p expression were associated with lower-grade and lower-stage tumors (Supp. Fig. 1E), while patients with high tumor EMBP1 expression were associated with higher-grade and higher-stage tumors (Supp. Fig. 1F).
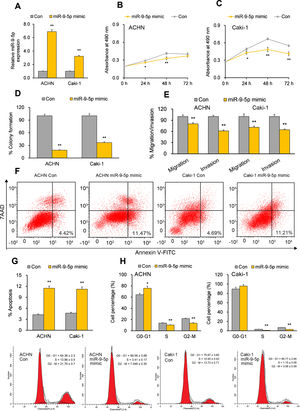
miR-9-5p displays tumor-suppressive effects in RCC cells in vitroTo establish the functional implications of miR-9-5p in RCC, we transiently transfected the RCC cell lines ACHN and Caki-1 with miR-9-5p mimic or a control mimic. RT-qPCR analysis 72hours after transfection showed the overexpression of miR-9-5p in the cell lines (Fig. 1A). Further functional analysis 72hours after miR-9-5p mimic transfection indicated reduced cell proliferation in both cell lines (Fig. 1B and C) and discernable reduction in their colony formation ability as compared to control (Fig. 1D). Cell migration and invasion capability of the RCC cells was also significantly reduced with miR-9-5p mimic transfection (Fig. 1E). Apoptosis analysis with FACS indicated that miR-9-5p mimic induced apoptosis in the RCC cell lines relative to control (Fig. 1F and G). A significant upsurge in the G0–G1 population and reduction in the S and G2–M populations was observed in ACHN cells (but not Caki-1 cells) overexpressing miR-9-5p (Fig. 1H).
Tumor-suppressive effects of miR-9-5p in RCC cells. ACHN and Caki-1 cells were analyzed 24 or 72h post-transfection with miR-9-5p mimic as compared with control miR. (A) miR-9-5p expression in ACHN and Caki-1 cells 72h post-transfection. (B, C) Quantification of cell proliferation in ACHN and Caki-1 cells 72h post-transfection. (D) Quantification of clone formation in ACHN and Caki-1 cells 72h post-transfection. (E) Quantification of migratory and invasive ability in ACHN and Caki-1 cells 72h post-transfection. (F, G) Annexin V-FITC/7AAD FACS quantification of early apoptotic cells (lower right quadrant) in ACHN and Caki-1 cells 24h post-transfection. (H) Cell-cycle analysis in ACHN and Caki-1 cells 24h post-transfection. In vitro experiments: n=3 biological replicates×3 technical replicates. Values are expressed as means±SEMs. *p<0.05, **p<0.01 vs. Con [Student's t-test].
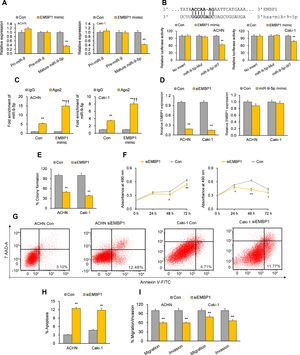
Previous bioinformatics research has suggested that EMBP1 may bind to and regulate miR-9-5p in RCC cells.10 By analyzing the effect of an EMBP1 mimic on miR-9-5p expression, we found a significant knockdown in the expression of mature miR-9-5p with no significant effects upon primary (pri)-miR-9 or precursor (pre)-miR-9 (Fig. 2A), suggesting that EMBP1-mediated regulation of miR-9-5p operates through a post-transcriptional mechanism. To verify if there was a direct interaction among EMBP1 and miR-9-5p, we used the in silico tool RNA22 (https://cm.jefferson.edu/rna22/) to identify miR-9-5p binding site(s) on EMBP1. A single binding site was identified in the EMBP1 sequence (Fig. 2B). To validate this binding, a luciferase reporter assay using miR-9-5p-WT sequence in ACHN and Caki-1 cells with EMBP1 mimic revealed suppressed luciferase activity (Fig. 2B). However, cells transfected with miR-9-5p-Mut sequence (containing a mutated binding site) showed no significant response to EMBP1 mimic, indicating a sequence-specific binding between EMBP1 and miR-9-5p. To further confirm their binding, an RIP assay was performed using Ago2 antibody and IgG control antibody in both RCC cell lines overexpressing EMBP1. These EMBP1 mimic cell lines exhibited increased fold enrichment in miR-9-5p within Ago2 versus IgG control (Fig. 2C), suggesting heightened integration of miR-9-5p into the miRNA ribonucleoprotein RNA-induced silencing complex (RISC complex) in response to EMBP1 overexpression.
EMBP1 directly regulates miR-9-5p; EMBP1 knockdown phenocopies miR-9-5p overexpression in RCC cells. (A) Effect of EMBP1 mimic on primary (pri)-miR-9, precursor (pre)-miR-9, and mature miR-9-5p expression in ACHN and Caki-1 cells 72h post-transfection. (B) Luciferase reporter assay in ACHN and Caki-1 cells 24h after co-transfection with miR-9-5p-WT or miR-9-5p-Mut plasmids and EMBP1 mimic or control mimic. (C) RNA immunoprecipitation (RIP) assay assessing miR-9-5p enrichment in Ago2 fraction with respect to IgG fraction in ACHN and Caki-1 cells 24h after transfection with EMBP1 mimic or control mimic. (D) EMBP1 expression following 25nM siEMBP1 (left) or 10nM miR-9-5p mimic (right) in ACHN and Caki-1 cells 72h post-transfection. (E–I) ACHN and Caki-1 cells were analyzed 24 or 72h post-transfection with siEMBP1 or control siRNA. (E) Quantification of colony formation in ACHN and Caki-1 cells 72h post-transfection. (F) Quantification of proliferation in ACHN and Caki-1 cells 72h post-transfection. (G, H) Annexin V-FITC/7AAD FACS quantification of early apoptotic cells (lower right quadrant) in ACHN and Caki-1 cells 24h post-transfection. (I) Quantification of migratory and invasive ability in ACHN and Caki-1 cells 72h post-transfection. In vitro experiments: n=3 biological replicates×3 technical replicates. Values are expressed as means±SEMs. *p<0.05, **p<0.01 vs. Con or IgG [Student's t-test].
With our studies showed a direct interaction between miR-9-5p and EMBP1 and EMBP1 negatively regulating miR-9-5p levels, we evaluated the functional implications of EMBP1 knockdown in RCC cells. The RCC cell lines ACHN and Caki-1 were transiently transfected with siRNA against EMBP1 (siEMBP1) and its expression was validated after 72h. Both the siEMBP1-treated RCC cell lines showed a significant knockdown in the expression of EMBP1 as compared to control (Fig. 2D). Previous research has suggested that miRNAs may reciprocally regulate the expression levels of regulatory lncRNAs (ref). When analyzing the effect of miR-9-5p mimic on EMBP1 expression, we found no significant effects upon the expression of EMBP1 (Fig. 2D), suggesting an absence of reciprocal regulation by miR-9-5p. Phenocopying miR-9-5p overexpression, EMBP1 knockdown inhibited colony formation and reduced cell proliferation relative to controls (Fig. 2E and F). It also promoted apoptosis (Fig. 2G and H) and reduced metastatic properties like migratory and invasive behavior (Fig. 2I).
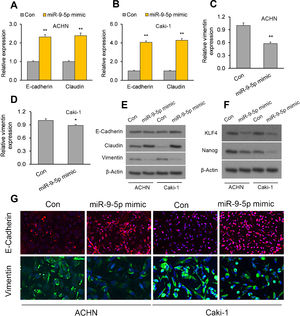
The EMBP1/miR-9-5p axis affects EMT and stemness markers in RCC cellsWe have demonstrated that EMBP1 directly regulates miR-9-5p expression and EMBP1 knockdown phenocopies the tumor-suppressive function of miR-9-5p. To evaluate whether this EMBP1/miR-9-5p axis affects EMT and stemness markers in RCC cells, RCC cells were transiently overexpressed with miR-9-5p, and we observed an upregulation in the epithelial markers E-cadherin and claudin and a simultaneous downregulation in the mesenchymal marker vimentin (Fig. 3A–E). Further, we performed immunofluorescence staining to study the localization of these marker genes under miR-9-5p-overexpressing conditions in ACHN and Caki-1 cell lines. We observed an induced E-cadherin expression (red) and enhanced localization to plasma membrane which is typical of epithelial cells (Fig. 3F, top), and reduced vimentin expression (green) was in the cell lines relative to control (Fig. 3F, bottom). Similar experiments were performed under EMBP1-silenced condition, and as anticipated, there was up-regulation in E-cadherin and claudin expression and a simultaneous down-regulation in vimentin expression (Supp. Fig. 2). Moreover, since the acquisition of EMT is shown to be mechanistically linked with a CSC-like phenotype, we also analyzed the expression of the stemness markers KLF4 and Nanog under miR-9-5p-overexpression conditions. We observed a decrease in KLF4 and Nanog protein levels in both ACHN and Caki-1 cell lines as compared with control, suggesting reduced stemness with miR-9-5p overexpression (Fig. 3G).
The EMBP1/miR-9-5p axis affects EMT and stemness in RCC cells. ACHN and Caki-1 cells were analyzed 72h post-transfection with miR-9-5p mimic as compared with control miR. (A–D) RT-qPCR analysis of (A, B) E-cadherin and claudin as well as (C, D) the mesenchymal marker vimentin in ACHN and Caki-1 cells. (E, F) Western blotting analysis of (E) E-cadherin, claudin, and vimentin proteins as well as (F) the stemness marker proteins KLF4 and Nanog in ACHN and Caki-1 cells. β-Actin was used as a loading control. (G) Immunofluorescence assays in ACHN and Caki-1 cells showing E-cadherin (red) and vimentin (green) immunostaining counterstained with DAPI (blue). In vitro experiments: n=3 biological replicates×3 technical replicates. Values are expressed as means±SEMs. *p<0.05, **p<0.01 vs. Con [Student's t-test].
Once we had demonstrated the effects of the EMPB1/miR-9-5p axis on RCC cells, we wanted to determine the effect of this lncRNAs/miRNA axis on the expression of downstream players that may be important in oncogenesis. Previous bioinformatics analysis on RCC tumor data has revealed that miR-9-5p may bind to and regulate CCNE2,20 an oncogene which is a key cell cycle regulator in RCC.21 Here, both RT-qPCR and Western blot analysis indicated miR-9-5p overexpression led to down-regulation in CCNE2 and downstream effector E2F1 (Supp. Fig. 3A and 3B). Accordingly, similar results were obtained following EMBP1 knockdown (Supp. Fig. 3C and D). These results highlight the importance of lncRNA-mediated regulation of tumor-suppressive miRNAs, which can serve to regulate important downstream effectors involved in EMT, stemness, and cell cycle control.
miR-9-5p mimic or EMBP1 knockdown inhibits RCC tumor development in mouse xenograft model, effects abrogated by CCNE2 overexpressionTo validate our in vitro results, we performed studies in two in vivo mouse ACHN xenograft models: (i) the first involved intra-tumoral miR-9-5p mimic therapy with/without CCNE2 rescue and (ii) the second involved intra-tumoral siEMBP1 therapy with/without CCNE2 rescue.
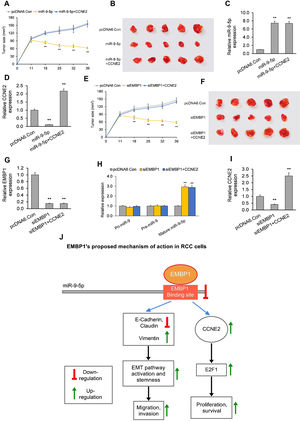
In the first model, when established xenograft tumors were locally administered miR-9-5p mimic, they showed a significant reduction in growth as compared to control tumors. The average tumor volume 39 days post-siRNA treatment was 63mm3 as compared to 158mm3 for control (Fig. 4A and B). RT-qPCR analysis of total tumor RNA from mimic only-treated tumors confirmed miR-9-5p upregulation and CCNE2 downregulation (Fig. 4C and D). RT-qPCR analysis of total tumor RNA from mimic+CCNE2-transfected tumors confirmed miR-9-5p upregulation and CCNE2 upregulation (Fig. 4C and D).
The EMBP1/miR-9-5p axis affects tumor growth in an in vivo mouse xenograft model. (A) Tumor volume analyzed after intra-tumoral injection of miR-9-5p mimic or control. pcDNA6-transfected ACHN xenograft tumors (with or without CCNE2 overexpression) were injected with miR-9-5p mimic or control at an interval of three days, starting from day 11 post-implantation. (B) Images of tumors on day 39 post-implantation. (C, D) RT-qPCR analysis on extracted tumor RNA for (C) miR-9-5p expression and (D) CCNE2 expression. (E) Tumor volume analyzed after intra-tumoral injection of siEMBP1 or control. pcDNA6-transfected ACHN xenograft tumors (with or without CCNE2 overexpression) were injected with siRNA or control at an interval of three days, starting from day 11 post-implantation. (F) Images of tumors on day 39 post-implantation. (G–I) RT-qPCR analysis on extracted tumor RNA for (G) EMBP1 expression, (H) primary (pri)-miR-9, precursor (pre)-miR-9, and mature miR-9-5p expression, and (I) CCNE2 expression. (J) Diagrammatic representation showing how EMBP1's negative regulation of miR-9-5p may promote pro-proliferative, migratory, and invasive pathways in RCC cells. Values are expressed as means±SEMs. *p<0.05, **p<0.01 vs. Con [Student's t-test].
In the second model, when established xenograft tumors in a mouse model were locally administered siEMBP1, they showed a significant reduction in growth as compared to control tumors (Fig. 4E and F). The average tumor volume 39 days post-siRNA treatment was 52mm3 as compared to 150mm3 for control. This reduction in tumor growth by EMBP1 knockdown was abrogated by addition of CCNE2 overexpression. RT-qPCR analysis of total tumor RNA from siEMBP1 only-treated tumors confirmed EMBP1 downregulation, mature miR-9-5p upregulation (with no significant effect upon pri-miR-9 and pre-miR-9), and CCNE2 downregulation (Fig. 4G–I). RT-qPCR analysis of total tumor RNA from siEMBP1+CCNE2-transfected tumors confirmed EMBP1 downregulation, mature miR-9-5p upregulation (with no significant effect upon pri-miR-9 and pre-miR-9), and CCNE2 upregulation (Fig. 4G–I), suggesting that CCNE2 overexpression did not significantly affect upstream EMBP1 or miR-9-5p levels.
DiscussionLncRNAs do not code for proteins but play a significant role in post-transcriptionally regulating gene expression in cancer cells.22 miRNAs have a well-established role in numerous malignancy-associated biological processes like cell growth, development, proliferation, and apoptosis.23 They can function as both oncogenic miRNAs (oncomiRs) or as tumor-suppressive miRNAs.24 Several studies have highlighted the roles of lncRNAs and miRNAs in RCC pathogenesis,25 but not much research has been done to highlight the crucial role their regulatory interaction may play in RCC pathogenesis. Therefore, although the tumor-suppressive role of miR-9-5p is well-known in certain cancers,13–15 the exact mechanism(s) by which miR-9-5p is regulated by lncRNAs in RCC needs to be elucidated.
In this study, we aimed at elucidating the functional significance of EMBP1 and miR-9-5p in RCC and understanding the mechanism by which they interact with each other and regulate metastasis-associated pathways. In this regard, we performed several in vitro and in vivo assays in RCC cell lines, patient tissue samples, and a mouse xenograft model. The expression levels of miR-9-5p in RCC cell lines and RCC tumor samples was low as compared to their respective controls. EMBP1, on the other hand, showed enhanced expression in both RCC cell lines and tissue samples as compared to controls. Our Chi-square analysis revealed significant associations between low miR-9-5p expression and high EMBP1 expression with advanced RCC tumor grading and staging, establishing their clinical significance in RCC.
Examining the functional implications of miR-9-5p overexpression in RCC cell lines revealed that miR-9-5p inhibited proliferation, clone formation, migratory behavior, invasiveness, and increased cell apoptosis alongside G0–G1 arrest. Similar functional changes were observed in RCC cells upon EMBP1 knockdown, suggesting that miR-9-5p overexpression or EMBP1 depletion can lead to suppression of RCC tumor progression. Now since the regulatory effects of lncRNAs are based their ability to bind and regulate the functioning of their RNA targets,26 we analyzed the binding ability of EMBP1 to miR-9-5p. Our in silico analysis using RNA22 revealed a single putative binding site in the EMBP1 sequence for miR-9-5p, indicating that miR-9-5p's tumor-suppressive functions may be negatively regulated by EMBP1. Interestingly, overexpressing EMBP1 in ACHN and Caki-1 cells produced a downregulation in miR-9-5p expression, further suggesting a possible direct interaction among them. To confirm this, a luciferase reporter assay confirmed a direct and sequence-specific binding between EMBP1 and miR-9-5p in ACHN and Caki-1 cells. This binding was further substantiated by a RIP assay, where the Ago2 immunoprecipitate showed enhanced enrichment of miR-9-5p in ACHN and Caki-1 cells overexpressing EMBP1 relative to control cells. This combined evidence supports a direct interaction between EMBP1 and miR-9-5p, wherein EMBP1 negatively regulates miR-9-5p.
The major obstacle in successfully treating advanced RCC tumors is their ability to reoccur and metastasize.27 The key functional process involved in the development of invasive and metastatic tumor cells is EMT, a conserved embryologic genetic program.28 During the process of EMT, there is a marked decrease in the epithelial markers E-cadherin and claudin, leading to disrupted cell-cell adhesion and acquisition of an invasive mesenchymal phenotype characterized by increased vimentin.29 In our study, overexpressing miR-9-5p in RCC cells lead to enhanced expression of E-cadherin and claudin along with a simultaneous decline in vimentin expression. Similar functional changes were observed upon EMBP1 knockdown; this is consistent with EMBP1's negative regulation of miR-9-5p. These phenotypic effects were in accordance with EMT inhibition, supporting a role for EMBP1/miR-9-5p axis dysregulation in RCC tumorigenesis.
Additionally analyzing whether EMBP1 regulation by miR-9-5p had any effect on further downstream pathway genes, we analyzed the expression of the predicted miR-9-5p target CCNE2 and its downstream effector E2F1, both of which are frequently altered oncogenes.30 CCNE2 hyperactivation leads to up-regulation in E2F1, leading to cell cycle dysregulation and cancer progression.30 We found both CCNE2 and E2F1 to be significantly down-regulated upon miR-9-5p overexpression in ACHN and Caki1 cells. Similar functional changes were observed upon EMBP1 knockdown; this is consistent with EMBP1's negative regulation of miR-9-5p. As these proteins play a significant role in dysregulating cell cycle progression,30 these findings also support a role for EMBP1/miR-9-5p/CCNE2 axis dysregulation in RCC tumorigenesis (Fig. 4J).
To establish our in vitro findings and the biological significance of reduced miR-9-5p expression, we carried out in vivo experiments in a nude mouse xenograft model of RCC. Local administration of siEMBP1 in xenograft RCC tumors produced an increase in miR-9-5p expression along with a significant attenuation in tumor volumes, an effect which was abrogated by CCNE2 overexpression. This in vivo data further validates EMBP1's negative regulation of miR-9-5p and supports a role for EMBP1/miR-9-5p/CCNE2 axis dysregulation in RCC tumorigenesis.
There are several limitations to this study. First, although we show that both EMBP1 and miR-9-5p are independently associated with advanced RCC tumor grade and stage, previous studies reveal that the kidney expresses relatively low levels of miR-9-5p31,32 and EMBP1.33 Our current results also support the low absolute expression of both miR-9-5p and EMBP1, which may impact the pathophysiological and clinical relevance of our findings. Second, although we show that EMBP1/miR-9-5p axis dysregulation affects the oncogene CCNE2 and CCNE2 overexpression eliminates the tumor-suppressive effects of EMBP1 knockdown or miR-9-5p overexpression, CCNE2 may not be the only factor by which EMBP1/miR-9-5p axis dysregulation promotes RCC tumorigenesis. Third, although we show that EMBP1 knockdown reduces xenograft RCC tumor volumes in vivo, cell line-derived xenograft models such as the ones used here may not represent the complexity and heterogeneity of human RCC due to the adaptation and selection of cell lines in vitro.34
Overall, the present work reveals a novel role of the EMBP1/miR-9-5p axis in affecting EMT and tumor progression in RCC. Moreover, both EMBP1 and miR-9-5p were independently associated with advanced RCC tumor grade and stage, suggesting their clinicopathological significance. Thus, our findings suggest an important role of the EMBP1/miR-9-5p axis dysregulation in RCC tumor progression. Future research in this field should focus on the downstream molecular pathway(s) affected by this EMBP1/miR-9-5p axis dysregulation in RCC tumors (such as the CCNE2/E2F1 pathway) and their clinical relevance in diagnosing, prognosing, and treating RCC patients.
Authors’ contributionsConceived and designed the study: YJH and YYL.
Performed the experimental procedures: YJH, YYL, ZQW, RZH.
Analyzed the data: ZMY, ZQW.
Drafted the manuscript: YJH.
Conflicts of interestNone.
This work was supported by the Zhejiang Basic Public Welfare Research Project (LQ19H050006). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.