The use of combination antiretroviral therapy has led to dramatic improvements in the life expectancy of HIV-infected persons. As result, the HIV population is aging and increasingly facing illnesses typically seen in the elderly, such as chronic kidney disease (CKD).
MethodsA retrospective longitudinal study was conducted using data from years 2010 and 2014 in all HIV-infected persons enrolled at the Spanish VACH cohort. We analyzed the prevalence and the predictive factors for developing CKD (estimated glomerular filtration rate, eGFR<60mL/min/1.73m2).
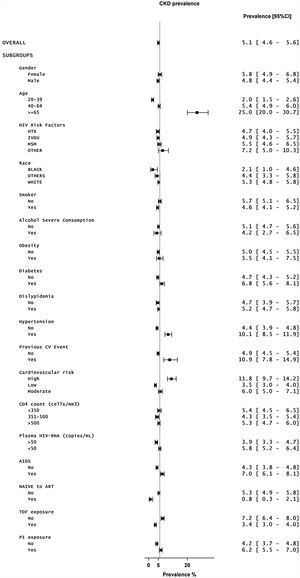
ResultsThe CKD prevalence at baseline was 456/8968, 5.1% [4.6–5.6%]. Of 8512 HIV-positive individuals examined without CKD at baseline (73.7% male, median age 44 years-old), 2.15% developed CKD (eGFR<60mL/min/1.73m2). The odds ratios [95%CI] for the independent predictive factors identified were gender (male) 0.54 [0.39–0.75], age (per year) 1.08 [1.07–1.10], AIDS diagnosis 1.40 [1.03–1.91], protease inhibitor-based regimens 1.49 [1.10–2.02], hypertension 1.37 [0.94–1.99], diabetes 1.84 [1.33–2.55] and history of cardiovascular events 1.66 [0.96–2.86].
ConclusionThe prevalence and risk factors for CKD and its progression are high in the VACH cohort. Thus, preventive measures such as control of hypertension, diabetes and obesity, as well as efforts for avoiding exposure to nephrotoxic drugs, including some antiretrovirals, are warranted in this aging HIV population.
El uso de tratamiento antirretroviral combinado ha dado lugar a mejoras sustanciales en la esperanza de vida de las personas infectadas por el virus de la inmunodeficiencia humana (VIH). Como resultado, la población con VIH está envejeciendo y haciendo frente cada vez más a enfermedades normalmente observadas en las personas de edad avanzada, como la nefropatía crónica (NC).
MétodosSe ha realizado un estudio longitudinal retrospectivo usando datos de los años 2010 y 2014 en todas las personas infectadas por el VIH incluidas en la cohorte VACH española. Se ha analizado la prevalencia y los factores predisponentes para el desarrollo de NC (filtración glomerular estimada [FGe]:<60ml/min/1,73m2).
ResultadosLa prevalencia de NC al inicio fue de 456/8.968; 5,1% (4,6-5,6%). De las 8.512 personas infectadas por el VIH evaluadas sin NC al inicio (73,7 varones, mediana de edad: 44 años), el 2,15% desarrolló NC (FGe<60ml/min/1,73m2). Los cocientes de posibilidades (IC del 95%) de los factores predictivos independientes identificados fueron 0,54 (0,39-0,75) para el sexo (varón); 1,08 (1,07-1,10) para la edad (por año); 1,40 (1,03-1,91) para el diagnóstico de sida; 1,49 (1,10-2,02) para los tratamientos basados en inhibidores de la proteasa; 1,37 (0,94-1,99) para la hipertensión; 1,84 (1,33-2,55) para la diabetes y 1,66 (0,96-2,86) para los antecedentes de acontecimientos cardiovasculares.
ConclusiónLa prevalencia y los factores de riesgo para la NC y su progresión son elevados en la cohorte VACH. Por lo tanto, está justificada la aplicación de medidas preventivas (como el control de la hipertensión, la diabetes y la obesidad), así como la aplicación de esfuerzos para evitar la exposición a fármacos nefrotóxicos (incluidos algunos antirretrovirales) en esta población con VIH que envejece.
Significant improvements in HIV therapies and increased antiretroviral treatment access have shifted HIV disease from being considered a life-threatening condition to become a chronic illness.1,2 This new paradigm brings new challenges in the management of the HIV population, as patients are living longer at the cost of prolonged exposure to antiretroviral drugs along with persistent immune activation and systemic inflammation associated with HIV infection.3 Accordingly, long-term drug toxicities and increased prevalence of age-associated comorbidities in the HIV population are currently a major focus of attention in HIV care.4,5 In this context, it is important to characterize the prevalence and risk factors for distinct age-associated comorbidities in the HIV population.
Certain antiretrovirals are associated with organ-specific toxicities, adding impact to other conditions that are age-related such as diabetes and hypertension.6 In this regard, chronic kidney disease (CKD) has become one of the major causes of death in the elderly HIV population living in Western countries.7 In the international D:A:D cohort, tenofovir disoproxil fumarate (TDF), ritonavir-boosted atazanavir, and ritonavir-boosted lopinavir were shown to be independent predictors of chronic renal impairment in HIV-positive persons.8,9 Likewise, in the EuroSIDA cohort that examined nearly 7000 HIV-positive individuals, exposure to TDF, boosted indinavir and boosted atazanavir were significantly associated with an increasing risk of developing CKD.10
Herein we report the rate and factors associated with renal impairment in a large population of HIV-infected individuals on successful antiretroviral therapy in Spain. We also tried to identify predictors for CKD in this aging HIV population. This knowledge should inform and improve the management of HIV care in general and choice of antiretroviral therapy in particular.
Patients and methodsThe VACH cohort is an open, prospective, multicenter cohort of HIV-infected adults consecutively attended since 1997 at 23 hospital-based HIV clinics distributed across Spain.11 Patients were included in the cohort provided that they were at least 16 years old and gave informed consent to participate in the study. All analyses conducted using the VACH cohort conform to Spanish laws and regulations regarding confidentiality, patient autonomy, data protection and medical research. Despite absence of a centralized laboratory, all of the VACH-associated clinics have used the same viral load tests and definition criteria for laboratory test abnormalities throughout the study period.
Information on factors associated with renal function was collected electronically, and definitions proposed by international guidelines were used.12 Arterial hypertension was defined as systolic blood pressure ≥140mmHg and/or diastolic blood pressure ≥90mm Hg or taking antihypertensive drugs. Grades 1 to 3 were used to stratify the severity of hypertension.12 Overweight and obesity were defined as BMI between 25 and 29.9, or >30kg/m2, respectively.12 Diabetes was defined as fasting glucose >140mg/dL or taking antidiabetic drugs or insulin.12 Dyslipidemia was defined as elevated total cholesterol ≥240mg/dL and/or elevated triglycerides ≥200mg/dL ever that year.12 High alcohol consumption was considered for a daily intake above 3 units (42g).12
Renal impairment was defined as an estimated glomerular filtration rate (eGFR) below 60mL/min/1.73m2 using the CKD-EPI formula13 that includes gender, ethnicity, age and creatinine values. Given that albuminuria was not considered for the definition of CKD, as it has been proposed by KDIGO guidelines,14 the current rate of renal impairment could be underestimated. We took the lowest measurement within a year when results from several tests were available. The severity of renal impairment was graded from 3 to 5 based, using thresholds in eGFR at 45, 30 and 15mL/min/1.73m2, respectively.12
The D.A.D. study group examined the rate and determinants of CKD in nearly 18,000 HIV-infected individuals and developed a score to predict the risk of developing CKD in HIV-infected patients.15
Data regarding the prevalence of renal impairment in the Spanish general population were provided by the EPIRCE study, an initiative of the Spanish Nephrology Society.16
Statistical analysisData from patients with available renal function at 2010 and 2014 were analyzed first in (a) a cross-sectional design for baseline prevalence description, and (b) a longitudinal retrospective design by excluding those patients with CKD at baseline for prospective prediction of CKD at 4 years of follow-up. We used absolute and relative frequencies for qualitative data, and median (interquartile range) for continuous variables. Confidence intervals for the prevalence estimation were calculated following the Wilson's method. Categorical variables were compared using the Fisher's exact test and continuous by means of the Mann–Whitney test. Uni- and multi-variate logistic regression analyses were performed for those variables found to be associated with kidney impairment in the univariate analyses with p values <0.10. The ROC-AUC was used for the selection of the best multivariate method.
Based on the distribution of the age in the VACH study population and the EPIRCE study used for comparisons, individuals were stratified into three categories (20–39, 40–64, and ≥65 years-old). All statistical tests were two-tailed considering a significance level of 5%.
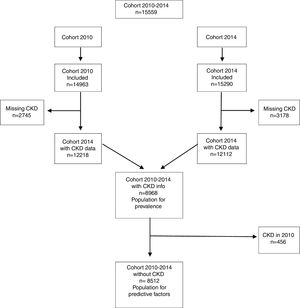
ResultsFrom a total of 15,559 individuals recruited in the VACH cohort until the end of 2014, 8968 had quality data to assess the renal function at baseline (year 2010) and after 4 years of follow-up (year 2014). The 8968 were analyzed for the cross-sectional part to describe the CKD prevalence and those without CKD at baseline, 8512, were included in the longitudinal part for the identification of predictive factors of CKD development (Fig. 1). Table 1 summarizes the demographics of the VACH study population.
Main features of the study population and univariate predictive factors for CKD.
| Total (n=8512) | Non-CKD (n=8329)* | CKD (n=183) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Male (gender) | 6274 (73.7%) | 6151 (73.9%) | 123 (67.2%) | 0.052 |
| Age (years) | 44 (39, 48) | 44 (39, 48) | 49.00 (44, 57) | <0.001 |
| Age groups (20–39/40–64/≥65 years) | 26.8%/71%/2.2% | 27.3%/70.8%/1.9% | 6%/80.3%/13.7% | <0.001 |
| Ethnicity (white/black/others) | 85.3%/3.3%/11.5% | 85.2%/3.3%/11.5% | 88%/1.1%/10.9% | 0.240 |
| Body Mass Index (kg/m2) | 23.9 (21.7, 26.5) | 23.9 (21.7, 26.5) | 24.2 (22.0, 27.2) | 0.105 |
| Scholarity (Primary/Secondary/University/Unknown) | 44.3%/20.8%/9.8%/25.1% | 44.2%/20.8%/9.8%/25.2% | 51.9%/18.6%/9.3%/20.2% | 0.196 |
| HCV | 3882 (45.6%) | 3797 (45.6%) | 85 (46.4%) | 0.245 |
| HBsAg | 459 (5.4%) | 443 (5.3%) | 16 (8.7%) | 0.102 |
| HIV parameters | ||||
| Risk group (IDU/MSM/Heterosex/Others) | 39%/24.7%/32.3%/4.1% | 38.9%/24.9%/32.2%/4.1% | 43.7%/15.3%/36.6%/4.4% | 0.032 |
| Time from HIV diagnosis (years) | 152 (71, 219) | 152 (71, 219) | 172 (85, 233) | 0.040 |
| AIDS diagnosis (%) | 2368 (27.8%) | 2299 (27.6%) | 69 (37.7%) | 0.003 |
| CD4 count (cells/mm3) | 541 (370, 744) | 542 (371, 744) | 519.50 (330, 710) | 0.087 |
| CD4 count (<350/351–500/>500cells/mm3) (%) | 21.9%/21.2%/54.3% | 21.8%/21.3%/54.3% | 26.2%/19.1%/51.9% | 0.532 |
| NADIR CD4 count (cells/mm3) | 188 (74, 300) | 189 (75, 300) | 140 (52, 270) | 0.004 |
| NADIR count (<350/351–500/>500cells/mm3) (%) | 82.2%/10.9%/6.7% | 82.1%/10.9%/6.7% | 85.8%/8.7%/5.5% | 0.551 |
| Plasma HIV-RNA (copies/mL) | 33 (19, 60) | 35 (19, 62) | 20 (19, 47) | 0.011 |
| Plasma HIV-RNA<50copies/mL | 5461 (64.2%) | 5319 (63.9%) | 142 (77.6%) | <0.001 |
| Naive to ART | 482 (5.7%) | 480 (5.8%) | 2 (1.1%) | 0.009 |
| Time on antiretroviral therapy (months) | 117 (42, 163) | 117 (42, 163) | 134 (70, 165) | 0.050 |
| Tenofovir-containing regimens* (%) | 57.0% | 57.0% | 55.2% | 0.648 |
| Protease inhibitor-based regimens | 43.2% | 42.9% | 54.1% | 0.003 |
| Classical risk factors for renal insufficiency | ||||
| Framingham cardiovascular risk | 7 (4, 13) | 7 (3, 12) | 12 (6, 22) | <0.001 |
| Framingham cardiovascular risk (low/moderate/high) | 54.2%/22.9%/8.2% | 54.6%/22.8%/7.8% | 35.5%/26.8%/23.5% | <0.001 |
| Hypertension (%) | 1028 (12.1%) | 978 (11.7%) | 50 (27.3%) | <0.001 |
| Diabetes (%) | 1491 (17.5%) | 1426 (17.1%) | 65 (35.5%) | <0.001 |
| Obesity (%) | 667 (7.8%) | 645 (7.7%) | 22 (12.0%) | 0.019 |
| History of cardiovascular events (%) | 271 (3.2%) | 253 (3.0%) | 18 (9.8%) | <0.001 |
| Dyslipidemia (%) | 6371 (74.8%) | 6214 (74.6%) | 157 (85.8%) | <0.001 |
| Current smoke (%) | 4938 (58.0%) | 4846 (58.2%) | 92 (50.3%) | 0.038 |
| High alcohol intake (>42g/day) | 5.10% | 5.10% | 4.90% | 1.000 |
| Alcohol (g/week) | 0 (0, 63) | 0 (0, 63) | 0 (0, 126) | 0.855 |
| COPD | 169 (2.0%) | 161 (1.9%) | 8 (4.4%) | 0.026 |
| Renal function | ||||
| eGRF EPI (≥90/≥60 to <90, mL/min/1.73m2) | 68.9%/31.1% | 69.9%/30.1% | 24.6%/75.4% | <0.001 |
| eGFR EPI (mL/min/1.73m2) | 99.01 (86.77, 106.99) | 99.33 (87.38, 107.09) | 75.39 (67.86, 89.96) | <0.001 |
| eGFR MDRD (mL/min/1.73m2) | 96.18 (83.38, 111.74) | 96.56 (83.73, 112.02) | 74.08 (67.30, 86.72) | <0.001 |
| eGFR MDRD (≥90/≥60 to <90, <60, mL/min/1.73m2) | 63%/36.6%/0.3% | 64%/35.7%/0.3% | 19.7%/79.8%/0.5% | 0.016 |
* Descriptive values are N, % or median (25th–75th percentiles).
These patients were seen at least once during that year. They represented the target population for this study. Overall, 74.6% were male and more than 90% were Caucasian. Their mean age was 46.1 years-old, and the majority (72.3%) was between 40 and 65 years-old. Only 4% of persons in the VACH cohort were older than 65 years-old.
Most HIV-infected persons in the VACH cohort had been diagnosed for an average length of 13.6 years. Their mean time on antiretroviral therapy was over 10 years. Overall 81.6% had undetectable plasma viremia and 62.1% had CD4 counts above 500cells/mm3. Up to 25.5% had experienced an AIDS-defining condition. Currently 55% were receiving TDF as part of their triple antiretroviral regimen and 42% were taking ritonavir-boosted protease inhibitors. Patients on both TDF plus boosted protease inhibitors as part of triple regimens represented 15% of the whole population. Anti-HCV antibodies were present in 43.6% of patients whereas serum HBsAg-positivity was found in 6%.
Conventional CKD risk factors were frequently found in the VACH cohort. Hypertension was present in 22%, diabetes in 16% and roughly 10% had obesity. The severity of hypertension was as follows: grade 1 (low) 71.9%, grade 2 (moderate) 21.4% and grade 3 (high) 6.7%. Dyslipidemia was found in 62% of patients, 30.4% had overweight, and 48% were current smokers. Prior cardiovascular disease (CVD) events were recorded in 4%.
As a reference, information from the EPIRCE study, a large Spanish HIV-negative population, was used. As expected, HIV-negatives were older than HIV-positives, with a mean age of around 50 years. Up to 26% of HIV-negatives were older than 65 years. Women represented 53%. Classical CVD risk factors were quite prevalent in the EPIRCE population, including dyslipidemia (29.3%), obesity (26.1%), hypertension (24.1%), diabetes (9.2%) and current smoking (25.5%). Despite being younger than the VACH population, rates of most CVD risk factors were similar or higher in HIV-positives, being roughly 2-fold greater for smoking and 3-fold higher for dyslipidemia.
The overall prevalence of renal impairment (eGFR<60mL/min/1.73m2) in the VACH cohort was 5.6% using CKD-EPI (6.1% using MDRD). As highlighted above, patients in the VACH cohort were significantly younger than in EPIRCE, and age was the strongest predictor of CKD in both studies. Of note, renal impairment was seen in 5.6% of HIV-infected individuals aged between 40 and 65 years-old but raised to 26.2% in older HIV-infected patients.
The severity of renal impairment split by grades 3–5 in HIV-positive patients from the VACH cohort using either CPK-EPI or MDRD is recorded in Table 1. It is noteworthy that the overall prevalence of CKD stages 3–5 (eGFR<60ml/min/1.73m2) was 45% using MDRD and 28.5% using CKD-EPI. In contrast, the rate reported in the general population, as in the EPIRCE study, was 6.8% using MDRD and 6.9% using CKD-EPI.17 For subsequent analyses in the VACH cohort, CPK-EPI estimates were only considered as they are more conservative and tend to be more accurate.18
Interestingly, as in HIV-negatives, women had increased rates of CKD compared to men (Table 2). As expected, moderate (grade 3) CKD severity (eGFR 30–60) was the most frequent in all age groups, rising to 21% in persons older than 65-years-old. It is noteworthy that CKD grade 5 (eGFR<15) or end-stage renal disease was only noticed in 0.3% of HIV-infected patients, but it rose to 1.9% in the subset of individuals older than 65 years-old.
Uni- and multivariate logistic regression analysis for predictors of renal impairment in the VACH cohort.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| p-Value | OR [95%CI] | AUC ROC [95%CI] | p-Value | OR [95%CI] | |
| Gender (male) | 0.044 | 0.73 [0.53–0.99] | 0.533 [0.499–0.568] | <0.001 | 0.54 [0.39–0.75] |
| Age (per year) | <0.001 | 1.09 [1.08–1.11] | 0.720 [0.683–0.757] | <0.001 | 1.08 [1.07–1.10] |
| AIDS diagnosis | 0.003 | 1.59 [1.17–2.15] | 0.551 [0.515–0.586] | 0.033 | 1.40 [1.03–1.91] |
| Protease inhibitor-based regimens | 0.003 | 1.57 [1.17–2.10] | 0.556 [0.519–0.593] | 0.010 | 1.49 [1.10–2.02] |
| Hypertension | <0.001 | 2.83 [2.03–3.94] | 0.578 [0.545–0.611] | 0.100 | 1.37 [0.94–1.99] |
| Diabetes | <0.001 | 2.67 [1.96–3.63] | 0.592 [0.557–0.627] | <0.001 | 1.84 [1.33–2.55] |
| History of cardiovascular events | <0.001 | 3.48 [2.11–5.76] | 0.534 [0.512–0.556] | 0.068 | 1.66 [0.96–2.86] |
| BMI (per 1kg/m2change) | 0.044 | 1.04 [1.00–1.07] | 0.537 [0.491–0.584] | ||
| HIV Risk Factors (Hetero) | 0.035 | 1 (Ref.) | 0.550 [0.512–0.587] | ||
| IVDU | 0.99 [0.71–1.37] | ||||
| MSM | 0.54 [0.35–0.84] | ||||
| Other | 0.94 [0.45–1.98] | ||||
| Plasma HIV-RNA <50copies/mL | <0.001 | 2.08 [1.44–3.01] | 0.571 [0.541–0.601] | ||
| Naive to ART | 0.016 | 0.18 [0.04–0.73] | 0.523 [0.515–0.531] | ||
| Framingham Cardiovascular Risk (High) | <0.001 | 1 (Ref.) | 0.627 [0.586–0.668] | ||
| Low | 0.22 [0.15–0.32] | ||||
| Moderate | 0.39 [0.26–0.60] | ||||
| Framingham Cardiovascular Risk (per 1 unit change) | <0.001 | 1.05 [1.04–1.07] | 0.662 [0.618–0.707] | ||
| Obesity | 0.021 | 1.71 [1.08–2.70] | 0.539 [0.508–0.571] | ||
| Dislypidemia | <0.001 | 2.06 [1.35–3.12] | 0.556 [0.530–0.582] | ||
| Smoker | 0.033 | 0.73 [0.54–0.97] | 0.540 [0.503–0.576] | ||
| COPD | 0.023 | 2.32 [1.12–4.79] | 0.512 [0.497–0.527] | ||
| Time since HIV diagnosis (per 12 months) | 0.038 | 1.02 [1.00–1.04] | 0.544 [0.502–0.587] | ||
| CD4 count (per 100cells/mm3) | 0.137 | 0.96 [0.01–77.59] | 0.538 [0.494–0.581] | ||
| NADIR CD4 count (per 100cells/mm3) | 0.025 | 0.90 [0.82–0.99] | 0.563 [0.520–0.606] | ||
| Plasma HIV-RNA (per 1 log change in copies/mL) | 0.091 | 1.00 [0.96 –1.04] | 0.555 [0.518–0.592] | ||
| Time on antiretroviral therapy (per 12 months) | 0.082 | 1.02 [1.00–1.05] | 0.542 [0.503–0.582] | ||
OR: odds ratio. AUC-CROC: area under curve of the receiver operating characteristic.
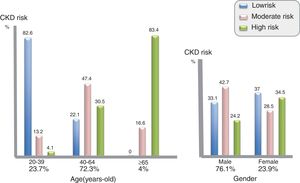
Using the risk score for CKD developed by the D:A:D study group, the proportion of HIV-infected patients in the VACH cohort with either low, moderate or high risk for developing renal impairment within 5 years was 34.1%, 39.2% and 26.7%, respectively. These figures are relatively high considering the average age of the population enrolled in the VACH cohort. In young HIV-infected persons (<40 years-old), the risk of CKD was 1.2% (0.2–4.7). In patients aged between 40 and 65 years, the risk of progressing to renal impairment was 7.7% (6.7–9.1). Finally, in older HIV-infected patients (≥65 years-old), the chance of developing CKD was 27.7% (22.6–33.3) (Fig. 2).
This is more clearly appreciated in Fig. 3, where D:A:D risk scores are represented for distinct age groups and gender. In univariate analysis, age was a stronger predictor of renal insufficiency (Table 2). As in HIV-negatives, women had a greater risk of developing CKD than men.
Besides age and gender, other classical variables were significantly associated with the development of renal impairment in HIV-positive patients. Table 2 records the impact of co-morbidities with the strongest association with CKD progression in the VACH cohort. In multivariate analysis, additionally including hypertension and body mass index, a prior history of CVD events, diabetes and dyslipidemia remained as independent predictors for developing renal impairment. Age and gender were excluded in this analysis because they were part of the CKD-eGFR definition.
DiscussionThis study is the first epidemiological investigation on the prevalence and risk factors of CKD in the Spanish adult HIV-infected population. Clinics across the whole country contributed to the cohort. The study population was large, roughly representing 15% of the estimated 100,000 individuals diagnosed with HIV that were living in Spain in year 2014, when this cross-sectional study was conducted.
The overall prevalence of CKD, as defined by an eGFR<60mL/min/1.73m2, was 5.6%, with significant differences according to age. Of note, the rate of CKD risen to 26.2% in HIV-infected persons older than 65-years old, although they only represented 4% of the VACH cohort. As reference, the rate of CKD in a representative sampling of 2746 HIV-negative adults in the general Spanish population was 6.9% in the EPIRCE study16; however, persons older than 65 years-old represented 26% of that cohort and women were 53%. Both older age and female gender are well-known predictors of CKD.14 Indeed, adjusting by age and gender, HIV-infected individuals in the VACH cohort had globally a higher rate of CKD than HIV-negatives.
Our findings are in agreement with results from other European studies. Indeed, the French multicenter Dat’AIDS cohort recently reported a rate of CKD of 18% in 1415 HIV-positive persons older than 60 years-old, being diabetes found in 14% and obesity in 12%, the most important risk factors. More importantly, CKD was associated with more than two-fold increase in 5-year mortality in this population.7 Likewise, in the large British CHIC cohort that examined 20,132 HIV-infected patients over 5 years,19 progression to advanced CKD stages 4–5 (<30ml/min/1.73m2) was significantly increased with reduced baseline eGFR, and patients with baseline eGFR<45ml/min/1.73m2 depicted an independent greater risk of death.
Given that antiretroviral therapy is currently recommended for almost all HIV-infected individuals,20 caution is warranted when using drugs known to be potentially harmful for the kidneys, such as TDF and/or ritonavir-boosted protease inhibitors.6,9,21 The improved life expectancy of HIV-infected individuals on antiretroviral therapy will inexorably led to a large and growing population of HIV-positive older patients within the next decades.4 In this regard, prioritization of those antiretroviral agents friendlier for the kidneys, such as the new formulation of tenofovir (tenofovir alafenamide, TAF) or abacavir-based regimes represent an important advantage.22 With TAF, reduced tenofovir plasma exposure would minimize tubular damage and bone mineral loss seen historically under long-term tenofovir therapy.23,24
Classical conditions associated with renal impairment, such as hypertension, diabetes, obesity and history of cardiovascular disease events5,14 were relatively frequent in our HIV-infected population, above what was seen in HIV-negative older Spaniards, as reported in the EPIRCE study.16 However, it was aging, a non-modifiable risk factor, that played the most significant role in the development of CKD in our patients with HIV infection.
A significant proportion of HIV-infected patients in our study had a high likelihood of developing CKD, as suggested by the D:A:D risk score.15 The global estimate was of 27% having a chance above 1:6 (>17%) of progressing to eGFR<60mL/min/1.73m2 within 5 years. Aware of these figures, efforts should be encouraged to better control modifiable CKD risk factors, such as hypertension, diabetes and obesity.5,25 It is mandatory to identify the subset of HIV-infected individuals with moderate to high risk for developing CKD, in order to implement as earlier as possible preventive measures for reducing the incidence of end stage renal disease and cardiovascular events. Furthermore, given the current and expected high burden of CKD in the Spanish HIV population, it seems crucial to avoid the use of nephrotoxic drugs such as some antiretrovirals or other drugs (especially non-steroid anti-inflammatory agents) with well-known renal safety issues. Opposite to other cohorts,9,10 we did not identify the exposure to tenofovir disiproxil fumarate as a risk factor for development of CKD.
Our study has inherent limitations, as it observational nature, retrieval of data by a heterogeneous group of physicians, missing results for important variables in a subset of patients, especially those referred to urine variables with impact in the assessment of CKD.12 However, the big set of data gathered may help to partially overcome all these facts.
In summary, in an increasingly aging western-world population, there is a high prevalence of CKD as well as risk factors for renal disease development and progression. Therefore, HIV caregivers must have great awareness of all potential nephrotoxic factors including age-associated comorbid conditions and drugs.
Conflict of interestThe authors declare no conflict of interest.
The study was funded by Gilead Spain. We would like to thank all VACH and CRETA participants, as well as Valeska Andreozzi (Exigo Consultores, Lisbon, Portugal) for her excellent scientific advice. Ferran Torres MD, PhD performed the statistical analyses.